Abstract
Ultrasound stimulation is promising as a non-invasive technique The present article examines various therapeutic techniques of ultrasound stimulation and its applications. These techniques are known not only as an effective tool in the treatment of diseases and health problems, but also for augmentation and rehabilitation.This article examines different types of ultrasound stimulations and their respective applications, the positive effects of these techniques and research results in different conditions and diseases.
Keywords: ultrasound stimulation; neuromodulation; non-invasive; therapeutic techniques; nervous system
Introduction
Ultrasound stimulation has various therapeutic applications. One of its applications in the field of neurology is neuromodulation. Neuromodulation represents a swiftly advancing domain within the field of neurotechnology, employing physical energy for the modulation of both the central and peripheral nervous systems. Neuromodulation techniques possess the capability to modify abnormal neural activity by employing electrical, magnetic, optical, or acoustic energy, consequently mitigating the disease condition and establishing stability within the system. Neuromodulation can be categorized into two main divisions: peripheral versus central nervous system (CNS) modulation and invasive versus noninvasive methods of interface [1]. Ultrasound neuromodulation is a non-invasive technique that can overcome the drawbacks of other neuromodulation techniques such as deep brain stimulation (DBS), transcranial magnetic stimulation (TMS), and transcranial current stimulation (tCS). Unlike DBS, it does not require surgery, which reduces the risk of infection and immunological reactions. Also, unlike TMS and tCS, ultrasound has better spatial specificity and can penetrate deep-seated brain regions, making it more suitable for targeting specific neural circuits. Therefore, ultrasound neuromodulation has the potential to be a safer and more precise alternative to other neuromodulation techniques [2],[3].
For understanding how ultrasound may affect the electrical activity of neuronal cells, the potential effects of non-electric stimuli such as ultrasound on the action potential (electrical signals) are clarified by biophysical models and hypotheses, including the soliton model, the flexoelectricity hypothesis, and the neuromembrane cavity stimulation model.
- Flexoelectricity Hypothesis
Like piezoelectricity, flexoelectricity is a phenomenon in which vibrations or mechanical changes in matter produce an electrical signal that leads to the occurrence of electric fluxes and related electrical effects. These electrical changes can increase or decrease the activity of neurons[4].
- Soliton model
The soliton model is a theoretical framework that describes how action potentials in nerve cells are produced and spread. According to this model, an ionic depolarization pulse that passes down the axon causes the action potential. The soliton model postulates that the action potential is a soliton, a self-reinforcing wave that keeps its shape and speed as it moves along the axon [5],[6]. According to the soliton model, thermodynamic factors can affect the action potential and it is a stable soliton.
- Neuronal intramembrane cavitation excitation (NICE) model
According to the Neuronal Intramembrane Cavitation Excitation (NICE) paradigm, ultrasound can affect neuronal activity by causing intramembrane cavitation to occur. When nanobubbles exist between the two leaflets of the lipid bilayer that make up the membrane of a neuron, this is referred to as intramembrane cavitation. According to this theory, the ultrasound’s mechanical energy is changed into electrical energy inside the neuron, causing modifications to the membrane’s electrical potential and the start of the action potential.
Finally, sound stimulation can have a variety of direct or indirect impacts on the function of neurons depending on the electrical processes and neurophysiology of neurons. Changes in membrane potential, action potential activity, and the transmission of nerve impulses in neural networks are only a few examples of these modifications [7]. We explained the basics of ultrasound in the previous article, you can visit our article [8] for more information about the biological effects of ultrasound, including cavitation. In this article,we will review the different techniques of ultrasound stimulation and the different applications of each technique in various fields, especially neuromodulation.
Types of ultrasound stimulation techniques and applications
Focused ultrasound Stimulation (FUS)
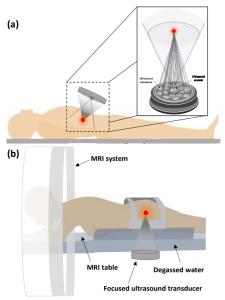
One prominent technique is focused ultrasound stimulation (FUS), which utilizes ultrasonic waves to target specific locations while minimizing pressure in surrounding areas. FUS can exert both thermal and mechanical effects, with the extent depending on the ultrasound exposure. Recent studies have demonstrated that both focused and unfocused ultrasound can modulate neuronal activity, although the patterns and outcomes of modulation differ. Focused ultrasound provides more effective energy delivery compared to unfocused ultrasound, which requires higher frequency and longer exposure for therapeutic impact. Transcranial focused ultrasound (tFUS), which is also a form of FUS, involves stimulating the brain through the skull in a non-invasive manner [2],[9],[10].
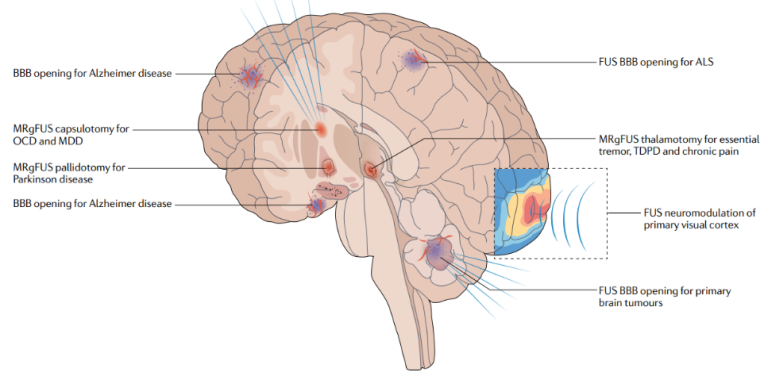
FUS has a wide range of applications in neurological and psychiatric conditions, such as Parkinson’s disease, ALS, epilepsy, reducing depression symptoms, enhancing memory performance in healthy individuals, chronic pain, and cognitive rehabilitation. For example, according to a recent study [11], FUS also is a promising method for non-invasive deep brain stimulation since it may have a good impact on the motivational and cognitive aspects of decision making. Applications in neurological and mental illnesses now cover the full clinical spectrum, and preclinical studies and human trials continue to yield new information. Figure 1, taken from the paper [9] illustrates the various intracranial applications of FUS in humans. Additionally, tFUS has been shown to activate specific brain regions, such as the hippocampus and the prefrontal cortex, which are involved in memory consolidation and decision-making, respectively.
In the following paragraphs, we will delve elaborate on some selected application of FUS.
- Open the Blood-Brain Barrier
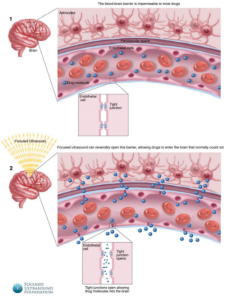
FUS has generated a great deal of interest as a safe treatment alternative because of its interactions with brain tissue, which range from blood-brain barrier opening (BBBO) to neuromodulation. In BBB, for example; the barrier is an obstacle to the passage of foreign bodies and actually keeps toxic substances away from the brain. As a result, it is also a barrier to keeping the drug away; here, FUS can be used to temporarily and safely open the blood-brain barrier to increase the transfer of drugs to the brain. This process is shown in Figure 2 [12].
- Parkinson
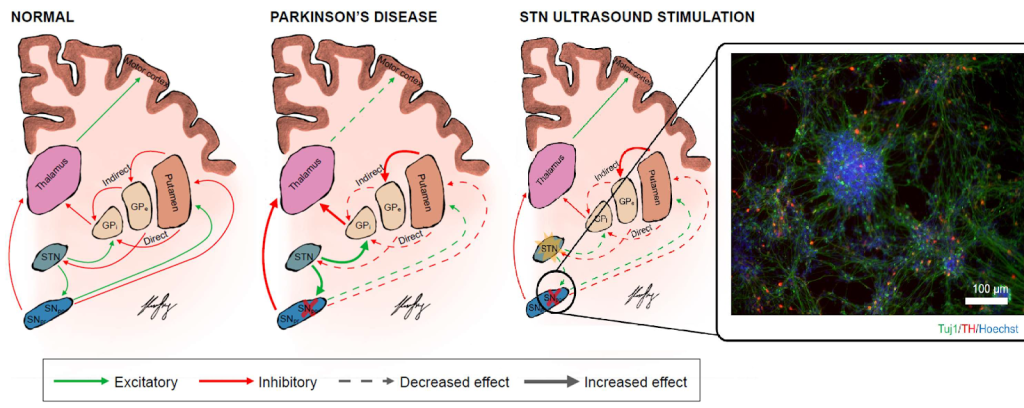
Modification of brain activity in patients with Parkinson’s disease (PD) using focused ultrasound stimulation (FUS) is described in study [13]. According to the paper, FUS is safe and has shown beneficial effects on motor behavior in PD patients. Moreover, FUS has sharper spatial resolution and non-invasive deep penetration, which makes it a promising tool for neuromodulating deep brain structures relevant to PD, such as the striato-pallido-thalamic network or deep structures including the STN and Gpi, as shown in Figure 3.
- Nerve pain control
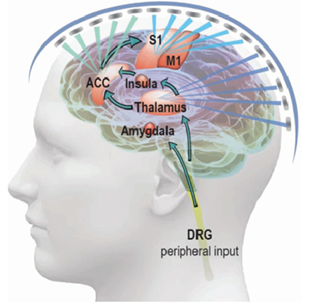
One kind of subjective experience that involves various functional brain circuits for processing is pain. Four ascending routes are involved in pain perception at the level of the central nervous system. These pathways carry nociceptive information from the dorsal root ganglions to the cerebral cortex [14]. Depending on the stimulation parameters and paradigm, tFUS modulation has been observed to produce both excitatory and inhibitory effects in the brain. tFUS has a higher spatial resolution than classic noninvasive brain stimulation methods like magnetic or electric stimulations and could stimulate deep brain areas. Because of this property, tFUS is an ideal tool for pain neuromodulation because it can almost target any region of the peripheral or central nervous system.
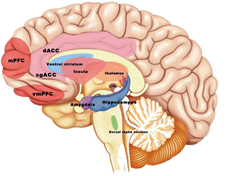
Pain associated with sickle cell disease (SCD) is an important model for chronic neuropathic pain syndromes in the central nervous system (CNS), due to its widespread nature and non-specific location. The ACC, S1, and insula are the most consistent locations, while the thalamus, ACC, and S1 are the most connected to pronociceptive areas in SCD patients with significant levels of pain. As shown in Figure 4, tFUS can be applied to these brain regions with specialized ultrasonic parameters and temporal sequences to suppress sensory input and promote emotional output. It is possible to increase neuromodulation effects while keeping spatial specificity by combining tFUS with pain-relieving therapies. Examples include endothelium selective transfection or ultrasound-enhanced therapeutic delivery by microbubble expansion, which provide spatially targeted medication or gene delivery. By incorporating pharmacological effects into the ultrasound neuromodulation framework, low-intensity tFUS can unlock and activate potential anesthetic agents, improving pain management capabilities [1].
Low-intensity focused ultrasound (LIFU)
Low-intensity focused ultrasound (LIFU) is a non-invasive technique that holds significant potential for neuromodulation in various clinical applications. It stands out due to its ability to penetrate the skull bone, concentrate in a targeted volume, and interact with biological tissues, making it a powerful tool for neuromodulation [15]. LIFU can also deliver low-intensity, pulsed mechanical waves to tissues like bone, cartilage, and tendon, resulting in regenerative and anti-inflammatory effects [2]. Its highly focused acoustic energy ensures a therapeutic effect only at the geometric focus of the transducer, making it precise and safe for treating neurological disorders [16],[17].
LIFU has been proven safe and capable of modulating neural tissue non-invasively in both animal and human subjects, targeting regions including the motor cortex, sensory cortex, visual cortex, frontal eye fields, and hippocampus. It can affect neuronal activity by causing the release of neurotransmitters like dopamine and glutamate and modulating the activity of ion channels [18],[2],[19]. It has been used to improve neuronal firing, suppress cortical and epileptic discharges, and induce behavioral changes in cortical and subcortical mammalian brain regions [20]. LIFU has shown effectiveness in thrombolysis in cerebral vasculature, urological surgery, neurosurgery, analgesic therapy, and other applications [15],[21]. The effects of LIFU on neuromodulation are believed to be attributed to thermal effects, radiation force, and acoustic cavitation [20].
- Blood-tumor barrier (BTB)
LIFU presents a promising alternative technique for functional neurosurgery, offering potential applications in tumor ablation [22],[23]. The combination of LIFU with intravenously administered oscillating microbubbles (MBs) has shown promise for systemic glioblastoma treatment. By enhancing the permeability of the blood-brain barrier (BBB) and blood-tumor barrier (BTB), LIFU+MBs facilitate the delivery of therapeutic agents directly to the brain or tumor parenchyma, potentially improving tumor control and overall survival [24].
Figure 5 illustrates the targeting and effects of low-intensity focused ultrasound (LIFU) during high and low pressures in the context of treating glioblastomas. At low pressure (stable cavitation), the red blood cells (RBCs) and immune cells are confined to the blood vessels, disrupting tight junctions (TJs) and creating openings with low levels of therapeutic extravasation. At higher pressure (inertial cavitation), wider openings are created between the endothelial cells, allowing for greater concentration of drugs delivered and extravasation of RBCs, immune cells, and therapeutic agents. The effects of LIFU targeting are visualized in Figure 5, which shows how external transducers target glioblastomas in practice and the possible scenarios at low and high pressures.
- Depression
Anxiety and depressive symptoms frequently coexist, and comorbidity is linked to more severe symptoms than either condition alone. Only 50% of patients who get standard first-line therapy experience remission, including those who receive psychotherapy and medication. Patients with depression have elevated levels of peripheral inflammatory biomarkers, and the degree of inflammation is correlated with the severity of particular symptoms. Multiple brain areas in depression patients exhibit neuroinflammation, according to research using positron emission computed tomography (PET) imaging and the 18 kDa translocator protein (TSPO) as a microglia biomarker (Fig.6). There is evidence that pharmacological therapy and neuromodulation interventions for psychiatric disorders can lower inflammation while providing symptomatic relief. TUS is one of the promising neuromodulation techniques that offers significant adjunctive therapy for the treatment of anxiety and depressive disorders [25]. Ultrasound stimulation has excellent therapeutic effects on depression in preclinical trials. Depressed model rats’ depressive behaviors were lessened by LIFUS stimulation of either the prefrontal cortex or the ventromedial prefrontal cortex (vmPFC), which was accompanied by higher levels of brain-derived neurotrophic factor (BDNF), whose downregulation is strongly associated with depression. An important finding was that LIFUS increased BDNF levels in the hippocampus of normal mice, pointing to a typical mechanism of BDNF signaling induced by ultrasonic stimulation. In addition, LIFUS improved adult hippocampus neural stem cells’ proliferation and neurogenesis [26]. Recent research by Sha-Sha Yi et al. [27] revealed that LIFUS can improve the behaviors of mice that have been depressed due to lipopolysaccharide. Additionally, LIFUS greatly decreased the increase in inflammatory cytokines caused by lipopolysaccharide. After around 30 minutes, healthy people’s moods were improved by tFUS targeting the right ventrolateral prefrontal cortex, according to research by Joseph L. Sanguinetti et al. [28]. Similar to this, research has indicated that LIFUS may elevate mood in healthy individuals [29]. These trials offer proof that LIFUS can be used to treat depression, but further research is still required to confirm that LIFUS actually improves the symptoms of depression.
High-Intensity Focused Ultrasound (HIFU)
This method involves the application of high-intensity ultrasound waves focused on a target location, raising the temperature to 70-80 °C. High-Intensity Focused Ultrasound (HIFU) is a technique used for non-invasive thermal or mechanical ablation of benign and malignant tissue. The strong heat generated by HIFU leads to the destruction of tissue through various mechanisms, including heat shock, tissue coagulation necrosis, and cavitation [30],[31],[32]. One notable advantage of HIFU is its ability to eliminate tissue with fewer adverse effects. Focal thermal ablation of deep-brain circuits, guided by magnetic resonance imaging (MRI), has shown promise in ameliorating movement abnormalities and psychiatric illnesses. Although the increase in temperature induced by ultrasound may be less visually prominent, temperature changes within the physiological range can still modulate neuronal activity [33].
- Tissue repair
In the context of wound healing, researchers have investigated the effects of High-Intensity Ultrasound (HIUS) on cellular processes involved in tissue regeneration. Their study utilized in vitro wound models and human dermal fibroblasts. The results showed that short-term ultrasound treatment significantly enhanced fibroblast proliferation, migration, and the production of crucial components for wound healing, such as fibronectin and collagen. Furthermore, HIUS promoted the contraction of a three-dimensional collagen matrix embedded with fibroblasts, particularly in the presence of TGF-β. RNA-sequencing and bioinformatics analyses revealed changes in gene expression and the activation of specific signaling pathways (p38 and ERK1/2 MAPK) in ultrasound-stimulated fibroblasts. These findings suggest that HIUS can activate human dermal fibroblasts, indicating its potential to improve wound healing and facilitate skin regeneration [33].
- Cancer
(HIFU) is a clinically applied technique for treating malignant and benign tumors. It offers several advantages over traditional cancer treatments such as chemotherapy, radiotherapy, and open surgery. HIFU is a non-invasive and non-ionizing modality that can precisely target tumors without harming the overlying skin and tissues. This is achieved by focusing the ultrasound energy at the tumor site, causing coagulative necrosis and effectively destroying the tumor tissue Figure 7. The focused energy causes coagulative necrosis of the tumor, effectively heating and destroying the tumor tissue. HIFU has been proven effective for treating both primary solid tumors and metastatic diseases, and its efficacy has been demonstrated in various cancer treatments. In liver cancer, HIFU has been utilized to selectively ablate tumor nodules, resulting in improved symptoms and extended survival periods for patients Figure 8 [34]. For breast cancer, HIFU offers a non-surgical approach, particularly beneficial for high-risk surgical patients. It provides advantages such as local tumor necrosis, reduced anesthesia requirements, shorter recovery times, lower infection risks, and no scar formation, with high rates of coagulation necrosis and disease-free survival observed. In prostate cancer, HIFU has shown promise by significantly reducing prostate-specific antigen levels and exhibiting positive survival rates, especially for patients who are not eligible for surgery. Similarly, HIFU ablation therapy presents an attractive alternative to surgery for small renal tumors, producing positive outcomes such as irreversible damage to treated areas, tumor necrosis, tumor shrinkage, and pain relief. HIFU therapy has also demonstrated potential efficacy in treating localized esophageal tumors, exhibiting significant tumor necrosis and improvement in dysphagia. However, when it comes to pancreatic cancer, although HIFU treatment has shown reductions in tumor size and response rates when combined with chemotherapy, complications and side effects have been reported, necessitating further research [35]. In the realm of brain cancer, HIFU has been studied for its feasibility in treating glioblastoma, the most common malignant brain tumor. It has demonstrated the ability to induce focal heating in brain tumors and possesses immunomodulatory effects that could potentially enhance immunotherapy response. Additional trials are required to validate these findings. Lastly, in bone cancer, HIFU has been used to destroy tumor microvessels, provide pain palliation for metastatic tumors, and treat primary bone malignancies [36],[37]. Treated patients have exhibited high survival rates, tumor regression, and pain reduction, highlighting the efficacy of HIFU in this domain.
![Figure 7: Schematic of the HIFU technology used for tumor therapy [34]](https://allostasis.io/wp-content/uploads/2023/06/HIFU-tumor-300x269.png)
Peripheral Focused Ultrasound Stimulation (pFUS)
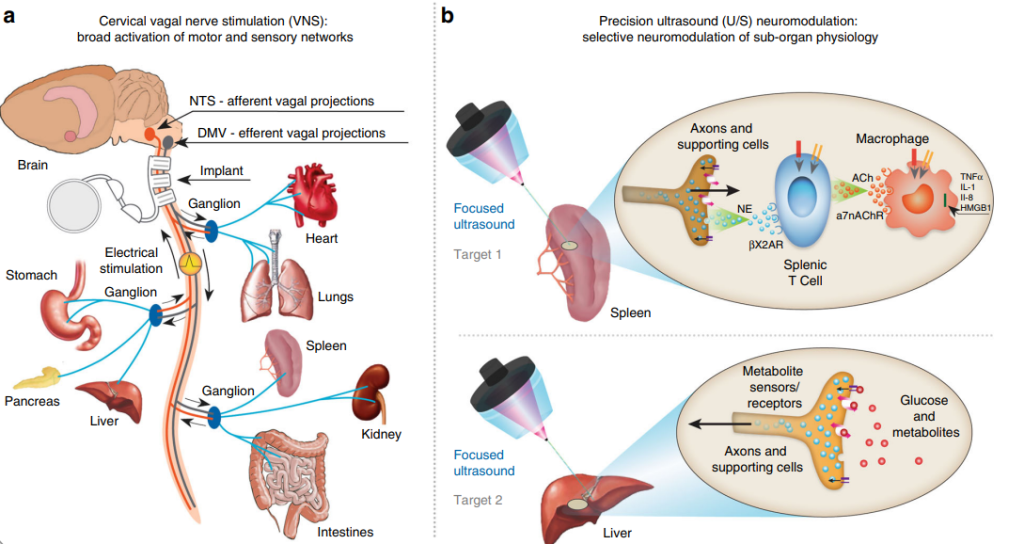
Peripheral focused ultrasound (pFUS) is another approach in ultrasound stimulation, providing precise stimulation to peripheral nerves, neural endings, or sub-organs. This method has emerged as a promising technique in neuromodulation and human-computer interaction. Two categories of pFUS exist: body-coupled ultrasound stimulation, commonly used for therapeutics or neuromodulation, and air-coupled contactless ultrasound haptic systems, enabling human-computer interaction paradigms Figure 9 [38]. Peripheral focused ultrasound (pFUS), offers high spatial and temporal resolution for targeted and localized stimulation without affecting surrounding tissues. This technique directly targets specific anatomical sites within organs, allowing for selective modulation of sub-organ physiology. Recent studies have investigated the effectiveness of pFUS in modulating neural signaling in peripheral organs such as the spleen and liver. These organs play crucial roles in regulating inflammation and blood glucose levels. The experiments have shown that pFUS can achieve similar outcomes compared to implant-based vagus nerve stimulation (VNS) [39]. Figure 10 visually illustrates the distinction between VNS and precision ultrasound (U/S) neuromodulation. In VNS Figure 10a, a device is implanted in the cervical vagus nerve, stimulating both target and non-target pathways. However, achieving precise stimulation by implementing smaller stimulators or advanced electrode designs closer to the target organ remains challenging. On the other hand, precision ultrasound neuromodulation Figure 10b directly targets specific anatomical sites within organs that are innervated by known axonal populations. In this case, the spleen and liver are targeted. The U/S technique allows for selective modulation of sub-organ physiology, such as the anti-inflammatory pathway in the spleen and metabolic pathways in the liver.
![Figure 9: Overview of Peripheral Focused Ultrasound (pFUS) Stimulation: Mechanisms, Parameters, and Applications [38].](https://allostasis.io/wp-content/uploads/2023/06/pFUS.png)
Low-intensity pulsed ultrasound ( LIPUS)
LIPUS (Low-intensity pulsed ultrasound) is a minimally invasive therapeutic technology that delivers acoustic pulsed energy to promote healing and regeneration in various parts of the body. It operates at a frequency in the low megahertz range, typically between 1 and 3 MHz. LIPUS has been widely recognized as an essential method for tissue repair and regeneration, particularly in the treatment of wounded tissues, bone healing, and wound healing [40],[41].
Research studies have shown that LIPUS has a significant impact on various cell types. By generating minor biomechanical interactions with cells, LIPUS triggers intracellular biological effects that ultimately contribute to tissue repair and regeneration. For instance, LIPUS stimulation has been found to positively affect stem cell-based tissue regeneration. Several types of postnatal mesenchymal stem cells (MSCs), including adipose-derived stem cells, bone marrow mesenchymal stem cells (BMSCs), periodontal ligament-derived stem cells (PDLSCs), and human umbilical cord-derived MSCs, have demonstrated increased viability, proliferation, and multilineage differentiation when exposed to LIPUS [41],[42].
In the dental field, LIPUS has shown promise in stimulating dental tissue regeneration. In vitro and in vivo research indicates that dentinogenesis, rapid periodontal tissue repair, and dental implant osseointegration can be facilitated by exposing dental tissues to LIPUS. One study specifically explored the therapeutic effect of LIPUS on dental and pulp tissue regeneration, utilizing mesenchymal stem cells from dental tissues. The researchers found that dental MSCs responded to LIPUS, leading to increased proliferation across various sources of MSCs. As well as, another study focused on the use of LIPUS to enhance the osteogenic potential of ligament stem cells extracted from the tooth’s side, showing increased osteogenic stage through pulsed ultrasound. These findings highlight the potential of LIPUS to control osteogenic capacity in an inflammatory environment through the unfolded protein response (UPR) in periodontal ligament stem cells (PDLSC) both in vitro and in vivo [43],[44].
LIPUS has also demonstrated effectiveness in improving bone fracture healing by inducing molecular, biochemical, and biomechanical changes at the fracture site. Compared to other fracture therapies, LIPUS therapy is considered a safe adjuvant therapy to accelerate bone healing in cases of new fractures, delayed fractures, and nonunions [45]. One specific study investigated the effects of LIPUS on the growth and osteogenic differentiation of human alveolar bone-derived mesenchymal stem cells (hABMSCs) for tooth tissue engineering. The research examined different duty cycles (20% and 50%) of LIPUS treatment and assessed cell viability, alkaline phosphatase (ALP) activity, gene expression, and mineralized nodule formation. The findings revealed that hABMSCs exposed to LIPUS exhibited higher cell viability, increased ALP activity, and upregulation of CD29, CD44, COL1, and OCN gene expressions compared to the control group. Moreover, LIPUS treatment enhanced mineralized nodule formation, suggesting its potential as an effective treatment for periodontal healing and regeneration [46].
Conclusion
We investigated the efficiency and appropriateness of ultrasound stimulation as a therapeutic, augmentation and rehabilitation approach. Due to its advantages, ultrasound stimulation can be a better alternative to other neuromodulation methods. According to the studies, we see that this non-invasive technique has a high potential in the development of neuroscience research and new treatments for psychiatric and neurological disorders. Also, the need for more research in this field is felt in order to examine the effectiveness and long-term effects of these techniques and the safety of these techniques on patients in more detail.
References
[1]. Kai Yu, Xiaodan Niu, and Bin He. “ Neuromodulation Management of Chronic Neuropathic
Pain in the Central Nervous System”. Adv. Funct. Mater. 2020, 30, 1908999. https://doi.org/10.1002/adfm.201908999
[2]. Haeyoung Joe, Ki Joo Pahk, Shinsuk Park, Hyungmin Kim “ Development of a subject-specific guide system for Low-Intensity Focused Ultrasound (LIFU) brain stimulation” ScienceDirect , Volume 176, July 2019. https://doi.org/10.1016/j.cmpb.2019.05.001
[3]. William J. Tyler, Yusuf Tufail, Michael Finsterwald, Monica L. Tauchmann, Emily J.Olson,Cassondra Majestic. “Remote Excitation of Neuronal Circuits Using LowIntensity, Low-Frequency Ultrasound”. PLoS ONE, October 29, 2008, doi:10.1371/journal.pone.0003511
[4]. Elisabetta Sassaroli, Natalia Vykhodtseva. “Acoustic neuromodulation from a basic science prospective”. Journal of Therapeutic Ultrasound volume 4, Article number: 17 (2016). https://doi.org/10.1186/s40349-016-0061-z
[5]. Andrew S. Johnson, William Winlow “The Soliton and the Action Potential – Primary Elements Underlying Sentience”. Front. Physiol., 25 June 2018 https://doi.org/10.3389/fphys.2018.00779
[6]. Revathi Appali, Ursula van Rienen, Thomas Heimburg. “Chapter Nine – A Comparison of the Hodgkin–Huxley Model and the Soliton Theory for the Action Potential in Nerves”. ELSEVIER Volume 16, 2012, Pages 275-299 https://doi.org/10.1016/B978-0-12-396534-9.00009-X
[7].Lemaire, Théo. Thesis : A modeling framework to understand and optimize ultrasound neuromodulation. Lausanne, EPFL. 2021
[8]. “The basic of ultrasound stimulation”
[9]. Ying Meng, Kullervo Hynynen , Nir Lipsman “Applications of focused ultrasound in the brain: from thermoablation to drug delivery”, Nature Reviews Neurology volume 17, pages 7–22 (2021). https://doi.org/10.1038/s41582-020-00418-z
[10]. Stuart Hameroff, Michael Trakas, Chris Duffield, Emil Annabi, M. Bagambhrini Gerace, Patrick Boyle, Anthony Lucas, Quinlan Amos, Annemarie Buadu, John J. Badal “Transcranial Ultrasound (TUS) Effects on Mental States: A Pilot Study”. Published by Elsevier Inc. May 30, 2012. DOI: https://doi.org/10.1016/j.brs.2012.05.002
[11].F. Munoz , A. Meaney , A. Gross , K. Liu , A.N. Pouliopoulos , D. Liu , E.E. Konofagou , V.P. Ferrera “Long term study of motivational and cognitive effects of low-intensity focused ultrasound neuromodulation in the dorsal striatum of nonhuman primates” . Published by Elsevier Inc. Brain Stimulation 15 (2022) 360e372 . https://doi.org/10.1016/j.brs.2022.01.014
[12]. https://www.fusfoundation.org
[13]. Keng Siang Lee, Benjamin Clennell, Tom G. J. Steward, Andriana Gialeli, Oscar Cordero-Llana, Daniel J. Whitcomb “Focused Ultrasound Stimulation as a Neuromodulatory Tool for Parkinson’s Disease: A Scoping Review”. Brain Sci. 2022, 12(2), 289; https://doi.org/10.3390/brainsci12020289
[14]. Lazzaro di Biase , Emma Falato , Maria Letizia Caminiti , Pasquale Maria Pecoraro, Flavia Narducci , and Vincenzo Di Lazzaro “Focused Ultrasound (FUS) for Chronic Pain Management:
Approved and Potential Applications”. Neurology Research International, 29 June 2021. https://doi.org/10.1155/2021/8438498
[15]. Elisabetta Sassaroli, and Natalia Vykhodtseva “Acoustic neuromodulation from a basic
science prospective” Journal of Therapeutic Ultrasound (2016).DOI 10.1186/s40349-016-0061-z
[16]. Anton Fomenko , Clemens Neudorfer, Robert F Dallapiazza , Suneil K Kalia , Andres M Lozano “Low-intensity ultrasound neuromodulation: An overview of mechanisms and emerging human applications”. Epub 2018 Aug 23. DOI: 10.1016/j.brs.2018.08.013
[17]. VISMAYA S. BACHU, JAYANIDHI KEDDA, IAN SUK, JORDAN J. GREEN and BETTY TYLER “High-Intensity Focused Ultrasound: A Review of Mechanisms and Clinical Applications”. Annals of Biomedical Engineering (2021). https://doi.org/10.1007/s10439-021-02833-9
[18]. Noah S. Philip, Amanda R. Arulpragasam “Reaching for the unreachable: low intensity focused ultrasound for non-invasive deep brain stimulation”. Neuropsychopharmacology volume 48, pages 251–252 (2023). https://doi.org/10.1038/s41386-022-01386-2
[19]. Ehsan Rezayat , Iman Ghodrati Toostani “Review Paper: A Review on Brain Stimulation Using Low Intensity Focused Ultrasound”. Neuroscience 2016
[20]. G.T. Clement “Perspectives in clinical uses of high-intensity focused ultrasound”. 2004 Elsevier. doi:10.1016/j.ultras.2004.04.003
[21]. Yi Xia , Jianhu Li, Disen Wang , Jinyun Chen , Mingxue Shen , Faqi Li , Yan Wang , Ping Jiang “Potential Application of Low-Intensity Focused Ultrasound in Rapidly Relieving Delayed-Onset Muscle Soreness Induced by High-Intensity Exercise”. J Ultrasound Med. 2022 Sep;41(9):2227-2235. doi: 10.1002/jum.15904
[22]. Amanda R. Arulpragasam, Mascha van ’t Wout-Frank, Jennifer Barredo, Christiana R. Faucher, Benjamin D. Greenberg and Noah S. Philip “Low Intensity Focused Ultrasound for
Non-invasive and Reversible Deep Brain Neuromodulation—A Paradigm Shift in Psychiatric Research”. Frontiers in Psychiatry ,24 February 2022. doi: 10.3389/fpsyt.2022.825802
[23]. Hongchae Baek, Ki Joo Pahk, Hyungmin Kim “A review of low-intensity focused ultrasound for neuromodulation”. Biomed. Eng. Lett. 7, 135–142 (2017). https://doi.org/10.1007/s13534-016-0007-y
[24]. Rajneesh Mungur , Jiesheng Zheng , Ben Wang , Xinhua Chen , Renya Zhan and Ying Tong “Low-Intensity Focused Ultrasound Technique in Glioblastoma Multiforme Treatment”. Frontiers in Oncology 19 May 2022. doi: 10.3389/fonc.2022.903059
[25]. Bingqi Guo, Mengyao Zhang, Wensi Hao, Yuping Wang , Tingting Zhang and Chunyan Liu “Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression”. Translational Psychiatry (2023) . https://doi.org/10.1038/s41398-022-02297-y
[26]. Scarcelli T, Jordao JF, O’Reilly MA, Ellens N, Hynynen K, Aubert I “Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice”. Brain Stimul. (2014) 7:304–7. doi: 10.1016/j.brs.2013.12.012
[27]. Yi SS, Zou JJ, Meng L, Chen HM, Hong ZQ, Liu XF, et al “Ultrasound stimulation of prefrontal cortex improves lipopolysaccharide-induced depressivelike behaviors in mice”. Front Psychiatry. (2022) 13:864481. doi: 10.3389/fpsyt.2022.864481
[28]. Sanguinetti JL, Hameroff S, Smith EE, Sato T, Daft CMW, Tyler WJ,et al. “Transcranial focused ultrasound to the right prefrontal cortex improves mood and alters functional connectivity in humans”. Front Hum Neurosci. (2020) 14:52. doi: 10.3389/fnhum.2020.00052
[29]. Hameroff S, Trakas M, Duffield C, Annabi E, Gerace MB, Boyle P, et al “Transcranial ultrasound (TUS) effects on mental states: a pilot study”. Brain Stimul.(2013) 6:409–15. doi: 10.1016/j.brs.2012.05.002
[30]. Florian Siedek, Sin Yuin Yeo, Edwin Heijman, Olga Grinstein, Grischa Bratke, Carola Heneweer, Michael Puesken, Thorsten Persigehl, Holger Grüll “Magnetic Resonance-Guided High-Intensity Focused Ultrasound (MR-HIFU): Technical Background and Overview of Current Clinical Applications (Part 1)”. Fortschr Röntgenstr 2019; 191: 522 – 530. DOI: 10.1055/a-0817-5645
[31]. Beatriz de Lucas , Laura M. Pérez , Aurora Bernal and Beatriz G. Gálvez “Ultrasound Therapy: Experiences and Perspectives for Regenerative Medicine”. Genes 2020, 11, 1086; doi:10.3390/genes11091086
[32]. Zahra Izadifar, Paul Babyn, Dean Chapman “Mechanical and Biological Effects of Ultrasound: A Review of Present Knowledge”. ultrasmedbio.2017.01.023 http://dx.doi.org/10.1016/j.
[33]. JeongYu Lee, Dae‑Jin Min, Wanil Kim, Bum‑Ho Bin, Kyuhan Kim and Eun‑Gyung Cho “Non pharmacological high‑intensity ultrasound treatment of human dermal fibroblasts to accelerate wound healing”. Scientific Reports | (2021) 11:2465. https://doi.org/10.1038/s41598-021-81878-1
[34]. Zahra Izadifar, Zohreh Izadifar , Dean Chapman and Paul Babyn “An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications”. J. Clin. Med. 2020, 9, 460; doi:10.3390/jcm9020460
[35]. Milka Marinova , Timo Wilhelm-Buchstab , Holger Strunk “Advanced Pancreatic Cancer: High-Intensity Focused Ultrasound (HIFU) and Other Local Ablative Therapies”. Fortschr Röntgenstr 2019; 191: 216–227. DOI https://doi.org/10.1055/a-0820-5564
[36]. Chuanxing Li, MD; Weidong Zhang, MD; Weijun Fan, MD; Jinhua Huang, MD; Fujun Zhang, MD and Peihong Wu, MD “Noninvasive Treatment of Malignant Bone Tumors Using High-Intensity Focused Ultrasound”. Cancer August 15, 2010. DOI: 10.1002/cncr.25192
[37]. Wenzhi Chen, Hui Zhu, Lian Zhang, Kequan Li, Haibing Su, Chengbin Jin, Kun Zhou, Jin Bai, Feng Wu, Zhibiao Wang “ Primary Bone Malignancy: Effective Treatment with High-Intensity Focused Ultrasound Ablation”. RSNA, 2010, https://doi.org/10.1148/radiol.10090374
[38]. Shi-Chun Bao, Fei Li, Yang Xiao, Lili Niu and Hairong Zheng “Peripheral focused ultrasound
stimulation and its applications: From therapeutics to human–computer interaction”. Frontiers in Neuroscience 14 April 2023. DOI 10.3389/fnins.2023.1115946
[39]. Victoria Cotero, Ying Fan, Tea Tsaava, Adam M. Kressel, Ileana Hancu, Paul Fitzgerald, Kirk Wallace, Sireesha Kaanumalle, John Graf, Wayne Rigby, Tzu-Jen Kao, Jeanette Roberts, Chitresh Bhushan,Suresh Joel, Thomas R. Coleman, Stavros Zanos, Kevin J. Tracey, Jeffrey Ashe, Sangeeta S. Chavan & Christopher Puleo “Noninvasive sub-organ ultrasound stimulation for targeted neuromodulation”. NATURE COMMUNICATIONS | (2019) 10:952 https://doi.org/10.1038/s41467-019-08750-9
[40]. Qianhua Gao, A. Damien Walmsley, Paul R. Cooper, Ben A. Scheven, “Ultrasound Stimulation of Different Dental Stem Cell Populations: Role of Mitogen-activated Protein Kinase Signaling”. 2016 American Association of Endodontists. Published by Elsevier Inc.
DOI: https://doi.org/10.1016/j.joen.2015.12.019
[41]. Bo Hu, Yuanyuan Zhang, Jie Zhou, Jing Li, Feng Deng, Zhibiao Wang, Jinlin Song “Low-Intensity Pulsed Ultrasound Stimulation Facilitates Osteogenic Differentiation of Human Periodontal Ligament Cells”. PLOS Published: April 17, 2014 https://doi.org/10.1371/journal.pone.0095168.g001
[42]. Ji Hao Cui, Kwideok Park, So Ra Park, and Byoung-Hyun Min “Effects of Low-Intensity Ultrasound on Chondrogenic Differentiation of Mesenchymal Stem Cells Embedded in Polyglycolic Acid: An in Vivo Study”. Tissue Engineering.Jan 2006.75-82. http://doi.org/10.1089/ten.2006.12.75
[43]. Gaoyi Wu, Lei Chen, Guoxiong Zhu, Yanliang Wang “Low-intensity ultrasound accelerates mandibular implant bone integration in dogs with mandibular osteoradionecrosis”. Journal of surgicalresearch April 30, 2012 . DOI: https://doi.org/10.1016/j.jss.2012.03.062
[44]. Saleh Al-Daghreer , Michael Doschak , Alastair J. Sloan , Paul W. Major ,Giseon Heo , Cristian Scurtescu , Ying Y. Tsui , Tarek El-Bialy “Long term effect of Low Intensity Pulsed Ultrasound on a human tooth slice organ culture”. archives of oral biology 57 ( 2 0 1 2 ) 7 6 0 – 7 6 8. doi:10.1016/j.archoralbio.2011.11.010
[45]. Poornima Palanisamy , Monzurul Alam , Shuai Li , Simon K. H. Chow , Yong-Ping Zheng “Low-Intensity Pulsed Ultrasound Stimulation for Bone Fractures Healing”. Journal of Bone and Mineral Research, 05 May 2021 .https://doi.org/10.1002/jum.15738
[46]. KiTaek Lim, Jangho Kim, Hoon Seonwoo, Soo Hyun Park, Pill-Hoon Choung, and Jong Hoon Chung “In Vitro Effects of Low-Intensity Pulsed Ultrasound Stimulation on the Osteogenic Differentiation of Human Alveolar Bone-Derived Mesenchymal Stem Cells for Tooth Tissue Engineering”. BioMed Research International Volume 2013, Article ID 269724, 15 pages
http://dx.doi.org/10.1155/2013/269724