Photobiomodulation Therapy in Peripheral Nerve System
Fateme Shirazian, Shirin Ahmadi
Abstract
Photobiomodulation has become a potential method for enhancing a variety of bodily biological processes over the past few decades. This safe and non-invasive technique has been investigated as a potential treatment for a variety of medical issues. In this article, we cover the present and potential uses of Photobiomodulation in the human body health and peripheral nervous system for augmentation , treatment, and rehabilitation, in addition to the science behind it. Also we bring up different kinds of biostimulation in wearable technology.
Keywords: Photobiomodulation, Photobiostimulation, low-level laser therapy, Rehabilitation, Rejuvenation, peripheral nervous system
1. Introduction
Photobiomodulation is a treatment method Based on studies demonstrating that red or near-infrared light radiation has a variety of physiological impacts on human and animal cells and tissues [1]. The first groundbreaking research in the field of medical phototherapy was done by Niels Ryberg Finsen in the late eighteenth century, using ultraviolet (UV) light to treat a variety of human illnesses. Endre Mester in 1967 offered the first proof of the possible impact of low-level laser therapy by using the ruby laser on the back of shaved mice implanted with tumor via an incision in the skin and since then, subsequent studies were conducted. Low level laser therapy was named for this type of treatment [2]. It was primarily employed to treat wounds, lessen pain, and reduce inflammation. Non-coherent light sources like light emitting diodes (LED) and broadband lamps have become widely used in recent years. Today, this procedure is known as photobiomodulation treatment [1]. Between 600 and 1100 nm, or from visible red light to near-infrared light, is the optical spectral range employed in LLLT (Low Level Laser Therapy)/PBMT (Photobiomodulation Therapy), and the light is often used with a maximum power density below 500 mW/cm2; over this value, tissue heating becomes troublesome [3]. The observed effects of PBM on more than a hundred various therapeutic indications may be related to its documented mitochondrial effects, despite the fact that medical treatments sometimes only have the ability to cure location-specific disorders. As oxidative stress and defective mitochondria have been linked to the majority of age-related chronic diseases, it makes sense that enhancing antioxidant defenses and mitochondrial function could help treat these conditions [4]. As a result of the shared metabolic origins of many chronic diseases, systemic therapeutic strategies that change metabolism may be able to address ailments in many distinct body areas [5].
In the continuation of this study, we review the mechanism and types of photobiomodulation treatment. Also, we examine its various medical uses, including its use in the treatment of peripheral nervous system diseases.
2. Production of Light (Electromagnetic Energy)
Light is the term used to describe the smallest unit of energy that may be transmitted in the form of elementary particles or waves. These particles are termed photons.This energy can only be seen as light, in a small range of the visible light of the electromagnetic spectrum, however electromagnetic radiation actually comprises of a wide variety of wavelengths and frequencies ,as shown in Figure 1.
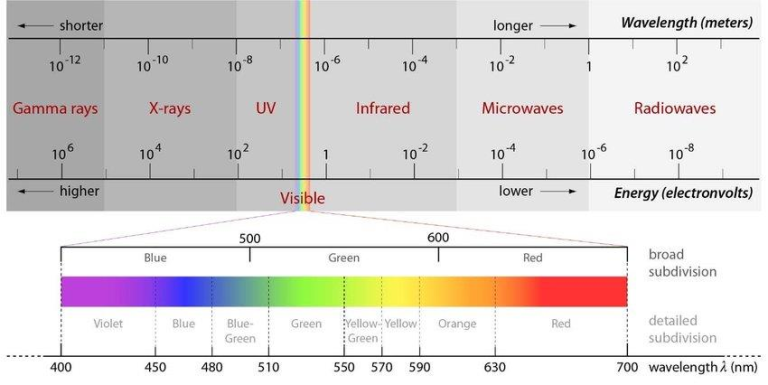
Since the development of quantum physics, light is currently seen to have a dual behavior: particle-wave. Light was first conceptualized as particles (quantum) or corpuscles (Isaac Newton’s theory), then as a wave ( The theory of Christiaan Huygens, Thomas Young, etc.), and finally as a duality behavior (particle-wave). All radiations and elementary particles in physics share the same properties: they all exhibit wave-particle duality, which means they can behave either as a particle or a wave or both, and they all move at the speed of light, but at various frequencies. It seems as though we must sometimes employ one theory and sometimes the other, while at times we may use either, said Einstein when attempting to explain the phenomenon of light.
During an electron transition in the atom, light (electromagnetic) energy is generated. In the electron transition phenomena, an electron absorbs energy from a single incident photon, utilizes some of this energy as kinetic energy to jump an orbit, and then releases some of the remaining excess energy as a photon on the way back to its original orbital position [6].
3. Mechanisms of Photobiomodulation
Different light wavelengths can be absorbed by numerous biological molecules. A photoreceptor, such as mitochondria, hemoglobin, or melanin, theoretically absorbs light [1].
The copper centers of cytochrome c oxidase (CCO), which are found at unit IV of the mitochondrial respiratory chain, are the primary chromophore to absorb red light in photobiomodulation with visible red light and near-infrared radiation. This results in the activity of numerous molecules including nitric oxide (NO), ATP, calcium ions, reactive oxygen species (ROS), and a variety of other signaling molecules [7]. To enhance electrons, certain near-infrared and red light wavelength ranges can be used. PBM is believed to stimulate electrons in chromophores to move from higher energy orbits, and then electron carriers (like the chromophore cytochrome c oxidase) deliver these electrons to their ultimate electron acceptors while a proton gradient is made, in addition to creating a proton gradient that boosts ATP production. This is thought to be the mechanism by which glycolysis and ATP production are promoted. Moreover, PBM activates a number of transcription factors [8]. Similar to photodynamic therapy, PBM promotes the generation of ROS (PDT).
Several studies have shown that after irradiation, mitochondrial membrane potential, oxygen consumption, and ATP levels all rise [9].
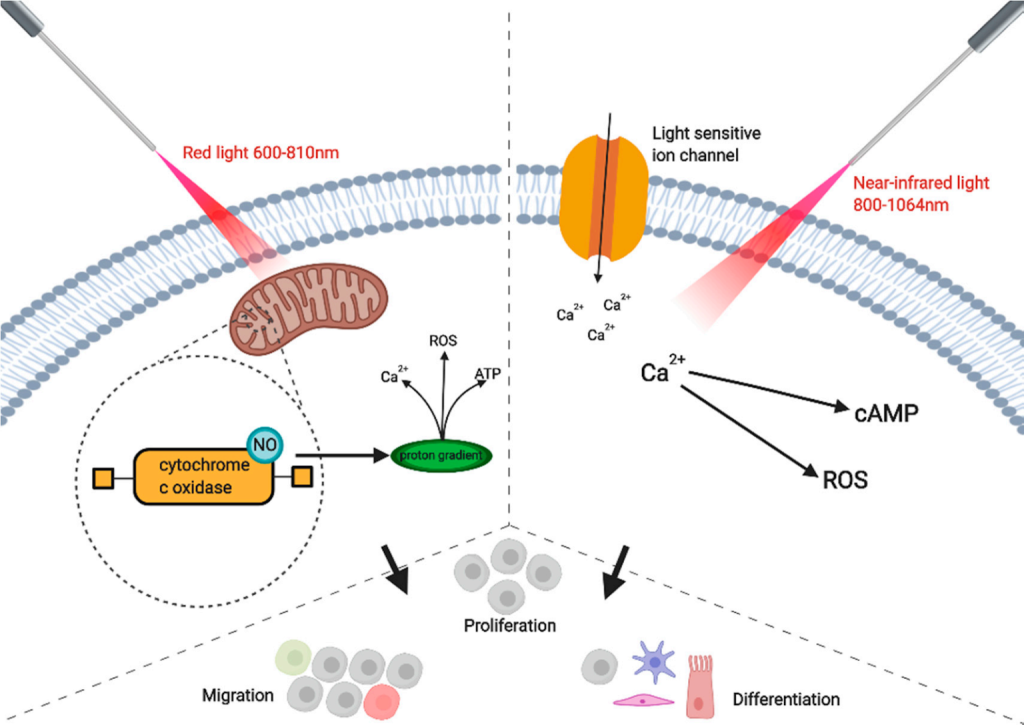
4. Types of Photobiomodulation
4.1. Laser
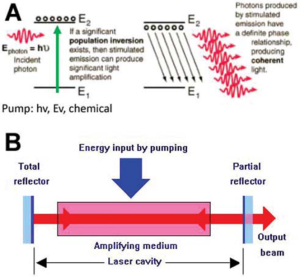
There are three ways that photons and electrons can interact: absorption of radiation, spontaneous emission, and stimulated emission (laser light). During absorption, electrons in a low energy state absorb energy from photons, causing them to move to a higher energy level. In spontaneous emission, the electron returns to its lower energy state and releases a photon of energy, resulting in ordinary, non-coherent light. Stimulated emission, on the other hand, occurs when an excited atomic electron interacts with an incoming photon of a specific wavelength, causing it to drop to a lower energy level and generate two identical photons that have the same phase, polarization, and frequency as the incident wave. These two photons travel in the same direction and are responsible for laser light [6].
Laser is an acronym for “light amplification by stimulated emission of radiation”. The fundamental working principle of a laser is the use of energy from light, electricity, or chemical reactions to pump electrons from a “laser gain medium” E1 to an excited state E2. After the majority of the electrons are in the excited state (population inversion), an entering photon (E photon) will drive the emission of a flood of fresh photons (coherent and polarised), and the light will then be amplified (Fig. 3A). In the laser building, the laser cavity has mirrors at either end that allow light to bounce back and forth while pumping occurs, greatly amplifying the light. To allow the laser beam to exit the cavity, one of the mirrors is only partially reflecting [10]. Based on the wavelength and frequency (Hz) of the laser light emitted, some lasers produce visible light while others produce invisible light [6].
4.1.1 Properties of Laser Light
Monochromaticity: Relates to its constant wavelength (λ). Laser light has a very limited frequency range. Monochromaticity is a decisive factor in biological tissue contact since it must be absorbed in order to interact with any tissue or substance. Light receptors (chromophores) in biological tissues are very selective to the wavelengths they absorb.
Polarization/directionality: This is another aspect of a laser that can influence how it interacts with matter. Different biological tissue or substance can absorb various light polarisations to various degrees. The wave oscillates in polarised light perpendicular to the direction of propagation (peak and trough are travelling linearly, in the same plane). The output of a laser is typically linearly polarized [11].
Coherence/in phase: This property allows for the concentration of a significant amount of power into a beam with a narrow spot size. It has been demonstrated that a pulsed coherent laser source can cause temporal evolution in the molecule, an incoherent thermal source of electromagnetic radiation cannot.
Intensity (brightness): determined in terms of the wave’s amplitude
Collimation: As rays travel over a distance, non-divergent, parallel rays provide the least amount of beam dispersion. The light beam diverges less as it moves across distance the more collimated it is. Collimated laser light doesn’t significantly lose energy while travelling and generally retains its beam spot size (diameter). A laser can concentrate a lot of energy in a tiny space (spot size). With non-collimated light beams, impossible to measure the energy dose supplied to the target. unless the beam is in close contact with the tissue.
4.1.2. Mode of Laser Radiation Emission
There are two primary types of light energy that laser systems may emit:
- Continuous wave (CW): Continuous average power and constant (or “on always”) emission.
- Pulsed or gated-pulse mode lasers (on and off emission): Pulsed wave lasers interrupt the emission of the laser light with pulses at various frequency (Hz) intervals and with various pulse lengths.The laser energy that they output changes on a periodic basis [6].
4.2. Light-emitting Diodes (LEDs)
LEDs are light sources made from semiconductor materials that exhibit the electroluminescence phenomena The most commonly used materials for this purpose are InGaN (60%) and AlInGaP (38%) [10]. It should be emphasised that the basic operating principle, known as the PIN semiconductor diode, is the same for both LEDs and diode lasers (Fig. 4). Electrons in the N (negative)-section and holes in the P (positive)-section separate when an electric potential is applied to the semiconductor. As these electrons and holes recombine in the I (intrinsic)-section, light is produced whose wavelength depends on the energy of the electrons. To generate a laser diode, a waveguide is added to the exterior of the PIN diode, which functions similarly to the mirrors in a conventional laser cavity[12].

4.3. Broadband Light
It is reasonable to assume that natural broadband light (blackbody radiation) originating from a heated object (such as a tungsten filament or the sun) could have similar biological effects if experimental evidence suggests that non-coherent and non-monochromatic light from LEDs can be used for photobiomodulation. There is still little but tentatively positive evidence for the good benefits of broadband light on PBM. The earliest publications on PBM date back to the very early 20th century, when a number of authors noted that incandescent lamps’ visible light, red, and infrared wavelengths appeared to have positive effects in the treatment of a variety of illnesses, including syphilis, smallpox, tuberculosis, chronic fatigue, diabetes, and obesity [10]. Wavelengths like visible light (400–800 nm) and water-filtered infrared A have been employed frequently (760–1400 nm) [13]. The majority of the results have been positive, and the effectiveness of the therapy seems to be on par with laser or LED photobiomodulation. It is possible to postulate that natural daylight or sunshine may also have health benefits connected to photobiomodulation given the apparent success of various broadband light sources [10].
5. Coherent and Incoherent Light in Photobiomodulation
Some of the characteristics of lasers, according to their supporters, may explain why PBM is better served by laser light than by LED light. The coherence feature is the one that is most frequently mentioned. Depending on the laser’s band-width, laser technologies produce coherent light with a range of coherence lengths. The He-Ne laser has a coherence length of several meters, while diode lasers have a coherence length of only a few millimeters. When coherent laser light interacts with tissue, small flaws in the tissue structure cause various phases to arise in each wavefronts, creating mutual interference patterns. The term “laser speckles” refers to these interference patterns, and the size of the speckles is proportional to the light’s wavelength. The size of a speckle in the visual range is less than one micron. One explanation contends that laser speckles are more effective in stimulating mitochondria than noncoherent LED light because subcellular organelles (such as mitochondria) have dimensions of this order [14].
Another very common assertion is that lasers penetrate deeper than LEDs. The power density at the surface and the depth into tissue at which a threshold power density is attained are closely correlated; therefore manufacturers assert deeper penetration [15]. A collimated laser beam is argued to be more likely to be forward scattered in tissue than a divergent LED beam. In addition to coherence, the bandwidth is a major difference between laser light and LED light. The bandwidth of a laser can be extremely small; for example, it can be a few hundredths of a nanometer for gas lasers and usually 1-2 nm for diode lasers [16]. On average, LEDs cost a lot less than laser technology. As a general rule, the cost per mW of optical power for LEDs is about a hundred times lower than lasers [17]. Since LEDs don’t need to be as safe as lasers, many quasi-monochrome LED-based home appliances are already accessible for individual use. Wearable technology can also be used with LEDs. Also, flat arrays made of many LEDs are possible. Because lasers often have modest to small spot sizes, this greatly expands the beam area, which makes it easier to treat broad body portions [18].
Due to the differences in important factors between the LED and laser groups, the majority of comparisons have a very high risk of bias, making it hard to draw valid conclusions about the performance of lasers and LEDs in photobiomodulation. However, the argument over whether lasers and LEDs are equivalent still reigns as the PBM field’s most contentious issue to this day [10].
6. Laser-Tissue Interaction
The optical characteristics of the tissue or substance that will interact with the particular light are just as important to know when designing a therapy procedure as the optical properties of electromagnetic radiation.
Depending on the optical characteristics of the receiver, light has diverse behaviours when applied to tissues or substances. The optical characteristics of the recipient will affect how light interacts with matter; light might experience absorption, scattering, penetration (transmission), and fluorescence (reflection). The optical properties that affect light absorption by the light receptors (chromophores), such as optical absorption and scattering coefficients, anisotropy, reduced scattering index, refractive index, light diffusion, and reflection of multi-scattered light, have all been tallied in numerous studies to predict the optical behaviour of biological tissues [ 11].
Additionally influenced by tissue geometries, such as superficial, interstitial, and within a cavity, optical characteristics have an effect on light fluence rate, which is a function of beam radius [19]. Laser absorption is also impacted by refraction, reflection, and backscatter.
Any “light” (electromagnetic radiation) therapeutic use requires that photons pass through the tissue and deposit their energy there through the optical absorption capabilities of that tissue. Its wavelength dependence is determined by the presence and quantity of different absorbing chromophores, such as blood components (haemoglobin, oxygen haemoglobin), water, melanin, fat, and yellow pigments (bilirubin, beta-carotene), in various types of tissues, as well as the tissue scattering properties [11]. Absorption and penetration may be affected by pigment specificity. Melanin, haemoglobin, and myoglobin were discovered to be strongly absorbent of short-wavelength visible laser light (between 400 nm and 700 nm) by Wilson and Jacques, whereas proteins and water are the main absorbents of infrared wavelengths. The effects of photobiological stimulation, according to Karu, depend on the wavelengths, dosage, and intensity of the light. Below, we outline the crucial variables affecting light-tissue interaction during laser therapy.
6.1. Laser variables: Laser Power, Light wavelength, Power Density of the Beam, Power Density and Energy Density of a Light Source, Light Delivery Protocol, Irradiation Spot Size, Top-Hat Beam Profile, Gaussian Beam Profile, Pulse Length, Pulse Repetition Rate, Light Delivery Pulse Modulation, Collimated or Diffuse Irradiation, Local Beam Angle of Incidence with the Tissue
6.2. Tissue variables: Thermodynamic Tissue Properties, Optical Properties of the Tissue, Tissue Temperature, Surface Contour, Tissue Index of Refraction, Tissue Blood Flow and Blood Content [6].

7. Current Applications of Photobiomodulation
7.1. Pain Management
Photobiostimulation accelerates the body’s natural processes for healing and pain relief. Although there are various theories, it is not fully understood how photobiostimulation reduces pain. According to one theory, photobiostimulation stimulates the ability of cellular mitochondria to produce ATP, a molecule that gives cells energy. A rise in ATP production may aid in accelerating healing and reducing pain because ATP is a molecule that gives cells energy [21]. According to a different notion, photobiostimulation could lessen pain by controlling the activity of nerve cells. It is specifically believed that the light may trigger the body’s natural painkillers, endorphins, to make more [22]. Also, there is research that suggests photobiostimulation may lessen inflammation [23,24].
As the immune system of the body reacts to an injury or infection, inflammation takes place and can lead to pain and swelling. This treatment may lessen pain by lowering inflammation. In illnesses like arthritis, musculoskeletal discomfort, and sports injuries, photobiostimulation can be utilized to decrease pain and improve performance [25]. In patients with subacromial impingement syndrome, Alfredo et al. (2021) [26] looked at the impact of LLLT paired with exercise on shoulder pain and impairment (SIS). LLLT paired with exercise significantly reduced pain and enhanced function over the course of three months, according to the authors’ analysis of the data.
A summary of the potential advantages of LLLT for pain management was provided by Dima et al. in 2017 [27]. They came to the conclusion that, in light of recent studies, using LLLT to treat pain and osteoarthritis disorders may be a complementary tactic utilized in clinical practice to help patients with osteoarthritis and chronic pain manage their symptoms. Low-level laser therapy’s impact on fibromyalgia patients’ pain, quality of life, and sleep may support the use of this treatment for those suffering from the condition [28]. According to studies, photobiostimulation can efficiently speed up healing and reduce pain following dental implant surgery [29]. There has been a significant rise in the number of postoperative uses for photobiomodulation (PBM) in oral and maxillofacial surgery (OMFS), including pain reduction [30]. PBM is also used in orthodontic treatment to lessen discomfort following the insertion of an orthodontic device [31,32]. Recently, Han et al. [33] observed that, when compared to a placebo group, facilitating healing considerably reduced pain (particularly the acute pain reduction). PBM has been demonstrated to have beneficial benefits in pain relief, healing acceleration, viral resistance mechanism inhibition, and recurrence reduction in herpes infections treated at levels below 10 J/cm2 [34]. When compared to the control group, PBM in the NIR (980 nm) with fluence of 4 J/cm2 was characterized as a helpful treatment for oral lichen planum [35].
7.2. Wound Healing
There are two categories of wounds: internal and exterior. Circulation problems, neuropathy, and medical conditions can all lead to internal wounds. The skin serves as a barrier to keep the body and environment apart. A burn, incision, or other external wound can be made when this barrier is broken. Homoeostasis, inflammation, proliferation and new tissue growth, as well as matrix creation and remodeling, are the four stages of wound healing that typically take place.
Pressure ulcers, diabetic ulcers, skin burns, and other types of wounds have all been treated with various dressings, growth factors, and other therapies, but many wounds are still resistant. Having a wound can have disastrous effects, including persistent pain, the loss of limbs or other bodily parts, and even death.
Application of photobiomodulation, either alone or in combination with other methods, is an alternate strategy that can assist balance wound moisture and encourage cell proliferation [1]. The inflammatory phase, a complicated protective reaction that involves transferring fluid, protein, and cells from blood to the wound site, can be lessened by laser therapy. By lowering neutrophil counts, oxidative stress, edema development, and numerous levels of biochemical markers, LLLT exerts anti-inflammatory effects. About 2-3 days after wounding, the proliferation phase is characterized by the development of granulation tissue, neovascularization, and epithelialization.
Electromagnetic radiation affects mitochondria, which use an electron transport chain to produce ATP. The biological processes of ATP, RNA, and DNA synthesis can be accelerated by LLLT, which also enhances the wound’s quality [36]. PBM therapy hastens the growth of new blood vessels and boosts the production of collagen, which promotes quicker healing and less scarring. Additionally, it lessens pain and swelling in the affected area. PBM therapy has also been demonstrated to have a favorable impact on the immune system, promoting the release of cytokines and growth factors that aid in the healing process.
In general, PBM therapy can encourage a quicker and more thorough healing process, improving outcomes for patients with different kinds of wounds. By reducing the chance of bacterial infection, laser therapy helps hasten the healing process. by de Oliveira et al, in a double-blind, placebo-controlled research, low-dose LED treatment (658 nm red) was utilized to heal second- and third-degree burn lesions. Following PBM treatments, all participants reported less discomfort and itching, decreased inflammatory exudate and fibrin, enhanced re-epithelialization, and better granulation tissue structure [37].
A singleblind, randomized clinical research was conducted by Taradaj et al. [38] to evaluate the efficacy of three widely used wavelengths for the treatment of pressure ulcers. The results showed that while the 808- and 940-nm treatments did not significantly improve healing rates relative to the placebo group, the 658-nm laser treatment was the most effective at encouraging wound closure.
Fitosa et al. and Mathur et al., in a study on diabetic foot ulcers, found that at 12 and 30 days after PBM treatment, wound size and pain scores were statistically lower in PBM-treated wounds than in the control group [39]. Simonovic et al, studying a human sample, claimed that for a group of patients treated with LLLT, significant wound healing, pain relief, and functional improvement were achieved compared to untreated patients.
670 nm is the second most used wavelength in LLLT, behind 632.8 nm. Other lasers, such as the 904 nm diode laser, the CO2 (10.6µm), Nd:YAG (1.064 µm), and ND:YLF (1.047µm), are frequently used as luminance. Other times, the treatment of wounds involves combining two or more distinct wavelengths. Short wavelength (632.8 nm) and long wavelength (904 nm). combination approaches account over 60% of the techniques used [1].
7.3. Rejuvenation and Beauty
7.3.1 Skin Rejuvenation
Applications of PBMT for the aesthetic treatment of disorders such fine wrinkles, scars, photoaged skin, inflammatory acne, and others have been under increased scrutiny during the past few decades. Moreover, PBMT has demonstrated a potential use for improving other dermatological disorders like vitiligo, acne, and hair loss [40].
LED lights are frequently used in dermatology and cosmetics applications to stimulate cellular metabolism and energy, which helps to produce collagen and elastin by increasing ATP synthesis and metabolising fibroblast cells. Low-intensity LED lights boost skin volume, minimise fine lines and wrinkles, and improve skin tone by passing directly through the epidermis of the skin and targeting the fibroblast cells that make collagen and elastin [41]. Also, the lights stimulate the release of NO and the energy transport system of cells, which increases localised blood flow and speeds up skin cell turnover to support healthy, resilient skin and promote growth stimulation and regeneration [42]. The appearance of aged/photoaged people has significantly improved under both yellow (590-595 nm) and red (630-633 nm) lightIncreased collagen production and fibroblast growth factor have been seen in skin treated with red light [43]. In other experiments, yellow LED lights have apparently proved successful in regenerating skin.
According to Kim et al. [44] , 595 nm yellow LED irradiation may result in an increase in cytochromes, a key target in fibroblast mitochondria, which subsequently triggers collagen remodeling . Moreover, using red LED light (633 nm) and infrared LED light therapy together has demonstrated a synergistic impact in the treatment of photoaged skin [45]. One well-known skin condition is acne vulgaris. The etiology of acne vulgaris, which results in the inflammatory lesions, is typically influenced by Propionibacterium acnes (P. acnes). Acne is treated safely and effectively with LED therapy, which the FDA recently approved.
The primary porphyrins that can absorb near-UV and blue light are porphyrins and coproporphyrin III. The most effective wavelength for treating acne is blue LED light (400–470 nm), which induces photodynamic death of P. acnes. Due to its weaker effect in activating coproporphyrin III but strong activator of protoporphyrin IX, red light is another ideal wavelength. For mild-to-moderate acne vulgaris, phototherapy with combined blue-red light is a painless, secure, and efficient treatment [41].
7.3.2. Hair Loss
Androgenetic alopecia is a hereditary type of hair loss that is caused by disruptions in androgen signaling, decreased proliferation of follicle epithelia, and shrinkage of terminal hairs on the scalp. red LED light (650 nm) has been shown to be an effective treatment for this condition. Red LED light (650 nm) can stimulate the hair bulbs, which are 2 mm beneath the dermal surface [46]. This stimulation increases cellular metabolism, improves capillary feeding, and reduces hair loss as a result. Additionally, red LED light also promotes the growth of new hair follicles via epidermal stem cells that are involved in hair follicle activation . This leads to an increase in the amount of hair on the scalp and reduction in inflammation brought on by cellular aging and daily stress conditions, ultimately reducing hair loss [47].
In a study conducted by Lee et al. (2018) [46] high-performance flexible red vertical light-emitting diodes (fVLEDs) were used in a hair-regrowth experiment with hair-loss mice to show the effectiveness of red LED light in stimulating hair growth. In comparison to the negative- and positive-control groups, the LED-light-treated group showed a tendency toward faster hair growth as well as a larger hair regeneration area after 20 consecutive days. The length of the photostimulated mouse hairs dramatically increased after 20 days. The photodynamic reaction that promotes hair development is enhanced by blue LED light, which also activates keratin and aids in the microbiological management of bacteria and fungi.
7.3.4. Cellulite and Fat Loss
Without causing any noticeable negative effects, LED light in PBMT has the potential to be employed for cellulite therapy and fat reduction [48]. The use of red LED light treatment, also referred to as “photonic lipolysis”, causes targeted fat cells to create tiny pores through which adipocytes’ internal lipids can be released.
Increased ROS levels were attributed to LED light therapy, which started the lipid peroxidation process. This reaction between ROS and cellular membrane lipids causes pores to form, momentarily breaks the membranes [41]. It is typically used in the beauty industry to treat the hips, thighs, and abdomen. Due to its therapeutic effects that enhance body aesthetics, revitalize the skin, and boost collagen synthesis, LED therapy is a crucial tool in the treatment of cellulite [49].
Paolillo et al. (2011) first reported the use of LED phototherapy during physical exercise to treat cellulite [50]. Based on a combination of high-intensity treadmill training and infrared-LED (850 nm) therapy, a new clinical procedure was created to enhance body aesthetics.
The infrared-LED improved skin texture and significantly reduced the circumference of the saddlebags and thighs by increasing microcirculation, lymphatic drainage, and collagen synthesis. To achieve the highest efficiency in fat loss, PBMT should be used in conjunction with exercise [51].
7.3.5. Tooth Whitening
In clinical dentistry, tooth appearance, particularly tooth color, has taken on greater significance. The most popular bleaching chemical has been high-concentration hydrogen peroxide (H2O2), although it has a number of drawbacks, including cytotoxicity, acute pulpalitis, gingival irritation, tooth sensitivity, and enamel demineralization. In recent years, phototherapy using a low-intensity light source from an LED/laser hybrid system has been investigated as a way to reduce the required concentration of H2O2 and accelerate the chemical reaction of the tooth-whitening process. As catalysts for ionization and the enhancement of the bleaching effect, LED light sources have a significant impact on tooth whitening.
Recently, Zhang’s team unveiled a novel polydopamine (PDA)-modified titanium dioxide nanoparticle tooth-whitening product (nano TiO2@PDA). After 4 hours of phototherapy and treatment, NanoTiO2@PDA activated by blue LED light demonstrated an ideal effect as a tooth-whitening agent, resulting in a ten-level whitening change. In contrast to treatment with 30% H2O2, it also demonstrated increased antibacterial activity without any discernible damage to the enamel surface. Due to its quick clinical process and increased bleaching effectiveness, blue LED light is frequently employed in tooth whitening [41].
7.4. Muscle and Joint Rehabilitation
Photobiostimulation has the potential to reduce the levels of inflammatory cytokines and other inflammatory markers, support tissue repair and regeneration through boosting ATP production, growth factor release, and the enhanced production of oxygen and nutrients in muscles. This can result in better muscle performance, less pain, and a quicker recovery from an accident or strain. PBM has been shown to be beneficial in the treatment of conditions such as arthritis, tendonitis, and sports injuries and can enhance the effectiveness of physical therapy and rehabilitation exercises. PBM’s effectiveness in treating human osteoarthritis of the knee and rheumatoid arthritis is well supported by research [48]. Skeletal muscle injuries account for more than 30% of accidents reported to medical facilities. An acute ischemic response to muscle injury results in the release of reactive oxygen species (ROS). These occurrences will ultimately lead to errors in DNA synthesis, protein oxidation, proteolysis, and cell membrane lipid oxidation.
Oxidative stress has been connected to a number of illnesses, such as diabetes, Alzheimer’s disease, and rheumatoid arthritis. On experimental muscle damage models, photobiomodulation treatment (PBMT) has been used to lessen the consequences of oxidative stress[49]. In patients with subacromial impingement syndrome (SIS), Alfredo et al. (2021) [26] looked at the impact of LLLT combined with exercise on shoulder pain and disability .
Based on their findings, scientists came to the conclusion that LLLT and exercise together reduced pain and enhanced function over the course of three months to a greater extent than either treatment group alone.
7.5. Neurological Conditions
Photobiostimulation for nerve regeneration has produced encouraging results in preclinical and clinical research. Recently, research has been done in the nervous system, especially for pain relief and nerve regeneration. It has been shown that PBM can support nerve regeneration, reduce inflammation, increase nerve conduction, improve mitochondrial function, and myelin formation. PBM has been used to treat neurological diseases in the central and peripheral nervous system and has had positive results [54].
8. PBM in Peripheral Nervous System
The peripheral nerves contain various nerve fibers for muscles, skin, vessels, joints, bones, and glands. A variety of factors, including cuts, crushing, direct impact, peripheral nerve stretching, nerve trapping in specific areas, intervertebral disc herniation, sometimes during joint dislocation and breaking bones, diseases such as diabetes and some syndromes, tumors, toxic substances, and sometimes due to drug complications, can lead to a peripheral nerve lesion. The intensity of the lesion on a peripheral nerve is divided into three groups: neuropraxia, axonotmesis, and neurotmesis. Studies have also suggested that photobiostimulation may be particularly effective when used in combination with other therapies, such as electrical stimulation or growth factors [55].
Human peripheral injuries are a significant clinical issue with inadequate care. These injuries frequently involve the upper limbs and are linked to a limitation with regard to performing daily living activities. PBMT is a promising method for promoting neural plasticity after nerve injury. Studies have investigated the effects of low-level laser therapy, light-emitting diodes, and other forms of photobiostimulation on various conditions, including diabetic neuropathy, carpal tunnel syndrome, and peripheral nerve injuries. The observed benefits include a decrease in inflammatory cells, a decrease in edema, an expedited healing of wounds, an increase in myelinated axons, a larger endoneural area, an expedited growth of axons, and an expedited growth of neurites in the dorsal ganglia [56].
Light can create a shift in the overall cellular redox potential toward more oxidation, higher ROS production, and cell redox activity. These aspects of oxidative stress are characteristic and are thought to play a role in the etiology of neurodegenerative disorders [57]. Glutathione, superoxide dismutase, and catalase are induced antioxidants in the PBM process that can improve the recovery of neurons by resuming mitochondrial function and restoring normal energy production. These effects can help promote nerve regeneration and improve nerve function.PBM can also cause local vasodilation by boosting nitric oxide release.
It has been noted that R/NIR laser light with wavelengths between 600 and 890 nm can penetrate up to 3 cm through skin and muscle to reach the injured region of various neurons. Light at 670 nm is more effective in reducing pain than light at 830 nm because CCO absorbs more at 670 nm [3].
According to the studies conducted, PBMT for the treatment of rat median nerve immediately after crush injury using LED (630 nm, 9 J cm2, 300 mW, and 0.3 W cm2) has positive effects on median nerve regeneration and muscle recovery [56].
The effects of two LLLT wavelengths (660 and 830 nm) on the regeneration of the sciatic nerve in rats were confirmed by Barbosa et al. [58], who discovered that LLLT at 660 nm with the same conditions led to a better functional recovery than the other groups. 17 publications examining the sciatic nerve, facial nerve, fibular nerve, vagus nerve, accessory nerve, alveolar nerve, and dorsal root validated the effects of PBMT on nerve injury. A wide range of application protocols for nerve lesions is available in the wavelength range of 632.8 to 904 nm, with good morphological, electrophysiological, immunological, and tissue marker results observed in experiments [55].
8.1. Carpal Tunnel Syndrome
Photobiomodulation therapy (PBMT)/Low Level Laser Therapy (LLLT), can be used to treat carpal tunnel syndrome. Carpal tunnel syndrome (CTS) is the most frequent entrapment neuropathy that leads to compression of the median nerve in the carpal canal generating sensory and motor symptom. PBM treatment for carpal tunnel syndrome typically involves applying low-level laser light to the wrist and hand area [59].
34 carpal tunnel syndrome sufferers were examined by Tezcan et al. [60] to see how they reacted to LLLT. This data showed that after LLLT, LLLT strain ratio and median nerve cross-sectional area decreased in patients. They arrived to the conclusion that patients’ situations were improved by the effects of laser therapy on nerve regeneration and the growth of the vascular supply .
A summary of several studies conducted for the feasibility of LLLT treatment in carpal tunnel syndrome is shown in Table 1 [61].The evidence indicates that the data is insufficient to infer any therapeutic effects of LLLT in the management of CTS.LLLT has a decreased effect in the treatment of CTS when given for a brief period of time, and clinically, pain is meaningfully reduced, according to poor to much reduced quality evidence.There is insufficient evidence to support LLLT having better or worse effects than alternative nonsurgical therapies in the management of CTS [61].
8.2. Neuropathic Pain
Studies have suggested that PBM can be a useful therapy for treating peripheral neuropathy, particularly for diabetic peripheral neuropathy.
PBM works by stimulating cellular metabolism, increasing blood flow, and reducing inflammation in the affected area, which can help to reduce pain, numbness, and other symptoms associated with peripheral neuropathy.
By mechanisms that include reducing histamine, serotonin, and bradykinin and enhancing acetylcholinesterase, aerobic metabolism, ATP, encephalin, and endorphin synthesis, PBMT could reduce acute neuralgia. Lasers have the potential to drastically raise the pain threshold and promote endorphin production [55]. Table 2 shows a summary of the sample of studies conducted in the treatment of various neurological diseases with photobiomodulation.
Table 1: PBMT/(LLLT) therapy in the intervention of CTS [ 61]
|
Authors |
TYPE OFINTERVENTION
USED |
Wavelength(nm) | Joules PER TREATMENT SPOT | MEAN OUTPUTPOWER ( mW) | RadiationAmount | NUMBER OFSPOTS
TREATED |
SESSIONS/SESSIONS PER
WEEK |
Intervention vs.Control programme | Outcome andAdverse effects |
| Barbosa,Rafael et
al.,[62] |
Gallium-indiumPhosphorus-aluminum
(AlGaInP) laser emitter |
660 | 10 | 30 | 20 | 6 | 12/2 | For 6 weeks,conservative
management along night splinting, educating patients and low-level laser therapy (12 therapy sessions) VS For 6 weeks, conservative therapy as night splinting and patient education was used. |
LLLT hasestablished result
as an intervention in patient with carpal tunnel syndrome. No clinically significant adverse effects. |
| Milicaetal.,[63] | GaAlAs diodelaser | 780 | 3.4 | 30 | 90 | 4 | 20/5 | All were treated oncedaily for two weeks
five times per week, then alternatively three weeks, for twenty sessions. |
They showeddecrease in pain
than the control group. No adverse effects were found. |
| Tikiz etal.,[64]60 | Gallium-Aluminum-Arsenide (Ga-AlAs) device | 830 | 1.5 | 30 | 60 | 5 | 15/5 | For three weeks, boththerapies were used
weekly five days. Practically and electrophysiological evaluations was done previously three, six, and twelve months following therapy. |
Pulsed US therapy, LLLT used and found significant positive impact in parameters. No clinically significant adverse effects |
Table 2: sample of Photobiomodulation Therapy (PBMT) studies in Peripheral
Nerve Regeneration [55]
| Authors | Type of Laser(Manufacturer) | Wavelength(nm)/Spot | BeamEnergy (mW) | EnergyDensity
(J/cm2) |
RadiationAmount | Variables | Irradiation Site | Evaluation Time | Main Results |
| Buchaim et al. [65] |
GaAlAs (Laserpulse IBRAMED, Brazil) |
660/0.116 | 30 | 4 | 16 s per point; 3 points | Sural nerve graft was coapted to thevagus nerve using the fibrin glue | Right side of the neck | Application on the 1st day post operatory, 5 weeks, 3 times a week. Evaluation 30 days after irradiation. |
LLLT improved the nerve regeneration. |
| Takhtfooladi et al. [66] |
InGaAlP (Teralaser; DMC®São Carlos, SP, Brazil) |
685/0.028 | 15 | 3 | 10 s per point | Crushing of the left sciatic nerve | On the surgery site on sciatic nerve | Application on the 1st day post-operatory, during 21 successive days |
LLLT accelerated and improved the nerve function after crushing lesion. |
| Wang et al. [67] |
GaAlAs (Transverse IND. CO., LTD., Taipei, Taiwan) |
808/3.8 | 170 | 38 15 |
67.2 s 179 s 335.6 s |
Crushing of the right sciatic nerve. | On lesion on sciatic nerve | Application during 20 successive days. |
LLLT (3 and 8 J/cm2) accelerated functional and morphologic recovery of the nerve, increased the expression of the marker GAP43. |
| Shen et al. [68] | GaAlAsP (Aculas-AM-100A, Konftec Co., Taipei, Taiwan) |
660/0.1 | 50 | 2 | 2 min per day; 2 points at the same time |
A biodegradable nerve conduit containing genipin-cross-linked gelatin was annexed using beta-tricalcium phosphate (TCP) ceramic particles (genipin-gelatin-TCP, GGT) with a 15 mm sciatic nerve transection gap. |
On the sciatic nerve. | Application 1st day post-operatory during 10 successive days |
LLLT accelerated the nerve regeneration due to the larger neural tissue, larger diameter and thicker myelin sheath, motor function, electrophysiology and muscular innervation. |
| Marcolino et al. [69] |
AlGaAs (Laser Diode, Ibramed) |
830/0.116 | 30 | 10 40 80 |
38.66 s 154.66 s 309.33 s |
Crushing of the right fibular nerve | On the right sciatic nerve | Application immediately after surgery and during the 21 successive days |
40 J/cm2 and 80 J/cm2 LLLT influenced the functional recovery of the nerve. |
| Sene et al. [70] | GaAsAl (Physiolux Dual, BIOSET, Rio Claro, Brazil) |
830/0.02 | 30 | 5 10 20 |
Maximum time of application was 40 s |
Crushing of the right fibular nerve. | Application fibular nerve region |
Application immediately after the lesion, during 21 successive days. |
LLLT simulation group obtained a larger nerve transverse area; group 10 J/cm2 obtained higher density of the fiber. LLLT did not speed up nerve recovery |
| Dias et al. [71] | GaAlAs (Mm Twin Laser Optics, São Carlos, Brazil) |
780/0.4 | 30 | 15 | 20 s per point; 3 points |
Latex protein (F1) on lesion per crushing of sciatic nerve |
On the surgery site, sciatic nerve. |
Application per 6 sessions on alternate days |
LLLT associated to the F1 protein did not present positive results and did not potentialize the effects of this protein. |
| Ziago et al. [72] |
GaAlAs (Twin Laser, MMO, São Carlos, SP, Brazil) |
780/0.04 | 40 | 4 10 50 |
4, 10 e 50 s per point; 3 points |
Crushing of the left sciatic nerve | On the surgical site | Application during 6 sessions on alternate days. |
Best morphological quantitative and morphometric results on L10 group after 15 days of nerve lesion. |
9. Wearable Devices in Photobiomodulation
9.1. Wearable PBM Patch
The wearable PBM patch has been developed to enable healthcare in real time while attached to the human body on a daily basis. It has a laminated structure of respective thin film components, allowing for a lightweight and a thin thickness of less than 1 mm. LED PBM devices are typically fabricated by arranging tens to hundreds of LEDs, so they have a weight of several hundred grams and a thickness of several centimeters. The wearable PBM patch consists of flexible OLED modules and a battery module with a patch to allow attachment to the human body. A multilayer thin-film encapsulation method was used to protect the OLED from moisture and oxygen, and a 75 µm flexible heat sink layer was laminated to ensure good thermal stability. wearable PBM patch that can be fastened to a person’s body for always-effective PBM. By maintaining equal power densities (>10 mW cm2) for each wavelength, the flexible OLED approach—not previously employed as traditional PBM lighting—allows free wavelength control in the 600–700 nm range .
The patch stimulates fibroblast migration and proliferation and has excellent effects on in vitro wound healing. Future therapeutic applications for it will include both medical and aesthetic procedures [73].

Figure 6. a) A 3D illustration shows an OLED PBM patch being applied to a person’s body for real-time medical monitoring. b) A picture of the patch applied to a person’s face along with illustrations of how the treatment might be used. c) A picture of the patch affixed to a human hand, along with illustrations of how the patch has been used in treatment. d) The wearable PBM patch’s structure. e) Driven by a patch without a separate power supply. f) Wearable PBM patch weight (0.8247 g) and thickness (676 m) measurements [73].
9.2. Hair Growth Helmet
There are three primary categories of LED hair loss treatment devices: hat-based (helmets or caps), hood-based, and brush-based (combs, hairbrushes, or headbands) [74]. Oaze is a PBMT helmet with a light source that emits LED light at 660 nm (2.5 mW, 18 units) and 630 nm wavelengths (3.5 mW, 24 units). Nevertheless, because it uses numerous LED modules and fiber optics, it is large, bulky, and complicated, and manufacturing it is challenging because multiple LEDs must be aligned [75]. Hamid [76], developed a special helmet that was granted a patent for the purpose of promoting hair growth and offering a healthier scalp, shinier hair, and denser hair. The flexible helmet’s LED array has 25–300 tiny LEDs that bio-stimulate the scalp and stimulate hair growth. A Japanese business recently developed the LED helmet HairRepro LED Premium as a novel way to regenerate hair growth utilizing red LED helmets. This LED helmet, created in Japan and now offered on the market, promotes hair growth by using 80 red LEDs with a wavelength of 630 nm. It is excellent for use in both beauty salons and at-home care [41].

9.3. Light therapy mask
To apply external LED light to biological tissue and the skin’s epidermis, LED masks have been produced. A phototherapy mask created by Stacy Francis and Oak Ridge in 2011 uses optical fibres connected to LEDs to irradiate and treat the epidermal skin area of a person’s face. For facial phototherapy treatments, in which the entire skin region of the face is exposed to radiation and treated, this LED mask technology offers a revolutionary mask. The iDerma firm has created an LED-based light-therapy mask that employs a whopping 142 LED probes that are both red and infrared. Using a progressive combination of 142 red and infrared LED lights with a specified wavelength of 660 nm and 940 nm, respectively, this unique device aids in improving the appearance of the skin.
Many red and infrared LEDs with both narrow-angle and wide-angle lenses, continuous delivery of therapeutic light therapy sessions, dial-adjustability to accommodate varied face sizes, and maximum comfort are just a few of its distinctive benefits [41].

10. Safety and Side Effects PBM
LLLT has been used for over 40 years and no long-term side effects have been reported. The most common side effect is mild skin irritation, which is usually temporary and can be reduced by adjusting the intensity or duration of light therapy. To ensure safety and effectiveness, it is important to use photobiostimulation devices that have been approved by regulatory agencies, such as the US Food and Drug Administration (FDA), as well as follow the manufacturer’s instructions for use. People with certain diseases such as eye diseases, Skin cancer or photosensitivity, pregnant women and children should also use photobiostimulation under the guidance of a healthcare professional. It is generally considered safe, but it is important to use it correctly and under the guidance of a healthcare professional to minimize the risk of side effects.
11. Conclusion
Considering the various medical applications that Photobiomodulation (PBM) has, it has a high potential in using biostimulation in wearable devices. Wearable PBM devices have the potential to provide a convenient and effective way to deliver light therapy for a wide variety of health and wellness applications. However, it is important to note that more research is needed to fully understand the optimal uses and effectiveness of these devices.
References
[1] Lau Pik Suan, Noriah Bidin, Chong Jia Cherng and Asmah Hamid “Light-based therapy on wound healing: a review”. Laser Phys. 24 (2014) 083001(12pp)
[2] Prof. Dr. med. Norbert Gretz Thesis “Impact of photobiomodulation on human skin melanocytes” Aufgelöst zum 16.04.2020
[3] Fatemeh Ramezani . Ali Neshasteh-Riz . Alireza Ghadaksaz. Seyedalireza Moghadas Fazeli. Atousa Janzadeh.Michael R. Hamblin . “Mechanistic aspects of photobiomodulation therapy in the nervous system”. Lasers in Medical Science :2021. doi.org/10.1007/s10103-021-03277-2
[4] JCamps and A. Garcia-Heredia, Introduction “oxidation and inflammation, a molecular link between noncommunicable diseases”, Adv. Exp. Med. Biol., 2014, 824, 1–4.
[5] E. Katsyuba and J. Auwerx, “Modulating NAD(+) metabolism, from bench to bedside, EMBO J., 2017, 36(18),2670–2683.
[6] Sonia Bordin-Aykroyd,Reinaldo Brito E1, William P Leavitt2, Galya Raz2 and Edward Lynch “Biophotonics: An introduction to New Laser Users” EC DENTAL SCIENCE August 20, 2019
[7] Hamblin, M.R. “Photobiomodulation for traumatic brain injury and stroke”. J. Neurosci. Res. 2018, 96, 731–743
[8] Claudia Dompe, Lisa Moncrieff, Jacek Matys , Kinga Grzech-Le´sniak, Ievgeniia Kocherova, Artur Bryja , Małgorzata Bruska, Marzena Dominiak, Paul Mozdziak , Tarcio Hiroshi Ishimine Skiba , Jamil A. Shibli, Ana Angelova Volponi, Bartosz Kempisty and Marta Dyszkiewicz-Konwi´nska: “Photobiomodulation—Underlying Mechanism and Clinical Applications” J. Clin. Med. 2020, 9, 1724
[9] T. H. Sanderson, et al., “Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain ischemia/reperfusion injury”, Sci. Rep., 2018, 8(1), 3481.
[10] Vladimir Heiskanena and Michael R. Hamblin “Photobiomodulation: lasers vs. light emitting diodes?” Photochem. Photobiol. Sci., 2018, 17, 1003
[11] Jacques SL. “Optical properties of biological tissues: a review”. Physics in Medicine and Biology 58.11 (2013): R37-61
[12] K. D. Desmet, et al., “Clinical and experimental applications of NIR-LED photobiomodulation” , Photomed. Laser Surg., 2006, 24(2), 121–128
[13] S. Dimitrios and L. Stasinopoulos, “Treatment of Carpal Tunnel Syndrome in pregnancy with Polarized Polychromatic Non-coherent Light (Bioptron Light)”. A Preliminary, Prospective, Open Clinical Trial, Laser Ther., 2017, 26(4), 289–295
[14] Z. Zalevsky and M. Belkin,”Coherence and speckle in photomedicine and photobiology”, Photomed. Laser Surg., 2011, 29(10), 655–656.
[15] J. T. Hashmi, et al., “Role of low-level laser therapy in neurorehabilitation”, PM R, 2010, 2(12 Suppl 2), S292–S305.
[16] K. D. Desmet, et al., “Clinical and experimental applications of NIR-LED photobiomodulation”, Photomed. Laser Surg., 2006, 24(2), 121–128
[17] H. S. Antunes, et al., “Cost-effectiveness of low-level laser therapy (LLLT) in head and neck cancer patients receiving concurrent chemoradiation”, Oral Oncol., 2016, 52, 85–90.
[18] M. R. Hamblin, et al., “Low level laser (light) therapy and photobiomodulation: the path forward”, in SPIE BiOS, SPIE, 2015.
[19] Sandell JL and Zhu TC. “A review of in-vivo optical properties of human tissues and its impact on PDT”. Journal of Biophotonics. 4.11-12 (2011): 773-787.
[20] Paromita Sarbadhikary*, Blassan P. George, Heidi Abrahamse “Paradigm shift in future biophotonics for imaging and therapy: Miniature living lasers to cellular scale optoelectronics” Theranostics 2022, Vol. 12, Issue 17
[21] Michael R Hamblin, “Mechanisms and applications of the anti-inflammatory effects of photobiomodulation”, AIMS Biophys. 2017 ; 4(3): 337–361. doi:10.3934/biophy.2017.3.337.
[22] D.M. FERREIRA , R.A. ZÂNGARO, A. BALBIN VILLAVERDE, Y. CURY, L. FRIGO, G. PICOLO, I. LONGO, and D.G. BARBOSA: “Analgesic Effect of He-Ne (632.8 nm) Low-Level Laser Therapy on Acute Inflammatory Pain”. Photomedicine and Laser Surgery
Volume 23, Number 2, 2005
[23] H OON C HUNG , T IANHONG D AI, S ULBHA K. S HARMA , Y ING -YING H UANG , J AMES D. C ARROLL , and MICHAEL R. H AMBLIN, “The Nuts and Bolts of Low-level Laser (Light) Therapy”. Annals of Biomedical Engineering (Ó 2011) DOI: 10.1007/s10439-011-0454-7
[24] Rodrigo Crespo Mosca, , Adrian A. Ong, Omar Albasha, Kathryn Bass, Praveen Arany: “Photobiomodulation Therapy for Wound Care: A Potent, Noninvasive, Photoceutical Approach”. ADVANCES IN SKIN & WOUND CARE & APRIL 2019
[25] Robert Dima, Vinicius Tieppo Francio, Chris Towery, Saied Davani, “Review of Literature on Low-level Laser Therapy Benefits for Nonpharmacological Pain Control in Chronic Pain and Osteoarthritis” Article in Alternative Therapies in Health and Medicine · October 2017
[26] Alfredo PP, Bjordal JM, Junior WS, Marques AP, Casarotto RA. “Efficacy of low-level laser therapy combined with exercise for subacromial impingement syndrome: A randomised controlled trial” Clin Rehabil. 2021;35(6):851-860. doi:10.1177/0269215520980984
[27] Dima R, Tieppo Francio V, Towery C, Davani S. “Review of Literature on Low-level Laser Therapy Benefits for Nonpharmacological Pain Control in Chronic Pain and Osteoarthritis” Altern Ther Health Med. 2017 Oct 2. pii: AT5647.
[28] Paulo de Tarso Camillo de Carvalho,Ernesto Cesar Pinto Leal-Junior, Ana Carolina Araruna Alves,Caroline Sobral de Melo Rambo, Luciana Maria Malosa Sampaio, Claudia Santos Oliveira, Regiane Albertini and Luis Vicente Franco de Oliveira “Effect of low-level laser therapy on pain, quality of life and sleep in patients with fibromyalgia: study protocol for a double-blinded randomized controlled trial” Trials 2012, 13:221
[29] Gianluigi Caccianiga, Letizia Perillo, Marco Portelli, Marco Baldoni, Cosimo Galletti, and Cosme Gay-Escoda “Evaluation of effectiveness of photobiostimulation in alleviating side effects after dental implant surgery. A randomized clinical trial” Med Oral Patol Oral Cir Bucal. 2020 Mar; 25(2): e277–e282.
[30] Sepanta Hosseinpour, DDS, MPH, PhD Student,1 Jan Tune´r, DDS,2
and Reza Fekrazad, DDS, PhD, FLD, FICD3,4 “Photobiomodulation in Oral Surgery: A Review” Photobiomodulation, Photomedicine, and Laser Surgery Volume 37, Number 12, 2019
[31] Artés-Ribas, M.; Arnabat-Dominguez, J.; Puigdollers, “ A. Analgesic effect of a low-level laser therapy (830 nm) in early orthodontic treatment” Lasers Med. Sci. 2013, 28, 335–341
[32] Genc, G.; Kocadereli, I.; Tasar, F.; Kilinc, K.; El, S.; Sarkarati, B. “ Effect of low-level laser therapy (LLLT) on orthodontic tooth movement”. Lasers Med. Sci. 2013, 28, 41–47
[33] Han, M.; Fang, H.; Li, Q.-L.; Cao, Y.; Xia, R.; Zhang, Z.-H. “Effectiveness of Laser Therapy in the Management of Recurrent Aphthous Stomatitis: A Systematic Review”. Scientifica (Cairo) 2016, 2016, 1–12.
[34] Merigo, E.; Rocca, J.-P.; Pinheiro, A.L.B.; Fornaini, C. “ Photobiomodulation Therapyn in Oral Medicine: A Guide for the Practitioner with Focus on New Possible Protocols”. Photobiomodul. Photomed. Laser Surg. 2019, 37,
669–680.
[35] Cafaro, A.; Arduino, P.G.; Massolini, G.; Romagnoli, E.; Broccoletti, R. “Clinical evaluation of the efficiency of low-level laser therapy for oral lichen planus: A prospective case series”. Lasers Med. Sci. 2014, 29, 185–190.
[36] PikSuan Lau a, Noriah Bidin a , Ganesan Krishnan a, Sana Mohammed AnaybBaleg a,Mohamad Bin Md Sum b, Hazri Bakhtiar a, Zaleha Nassir c, Asmah Hamid d: “Photobiostimulation effect on diabetic wound at different power density of near infrared laser”. Journal of Photochemistry and Photobiology B: Biology 151 (2015) 201–207
[37] De Oliveira RA, Boson LLB, Portela SMM, Filho ALMM, de Oliveira Santiago D. “Low intensity LED therapy (658 nm) on burn healing: a series of cases”. Lasers Med Sci 2018;33(4):729-35.
[38] Taradaj J, Halski T, Kucharzewski M, Urbanek T, Halska U, Kucio C. “Effect of laser irradiation at different wavelengths (940, 808, and 658 nm) on pressure ulcer healing: results from a clinical study”. Evid Based Complement Alternat Med 2013;2013:960240.
[39] Feitosa MC, Carvalho AF, Feitosa VC, Coelho IM, Oliveira RA, Arisawa EAˆ . “Effects of the low-level laser therapy (LLLT) in the process of healing diabetic foot ulcers”. Acta Cir Bras 2015;30(12):852-7
[40] M.K. Sawhney, M.R. Hamblin, “Low-level light therapy (LLLT) for cosmetics and dermatology, Proc”. SPIE 8932 (2014) 1–12.
[41] Vinh Van Tran a,b, Minhe Chae c, Ju-Young Moon d,*, Young-Chul Lee b: “Light emitting diodes technology-based photobiomodulation therapy (PBMT) for dermatology and aesthetics: Recent applications, challenges, and perspectives” Optics & Laser Technology 135 (2021) 106698
[42] A. Jahan, M.A. Nazari, J. Mahmoudi, F. Salehpour, M.M. Salimi, “Transcranial near-infrared photobiomodulation could modulate brain electrophysiological features and attentional performance in healthy young adults”, Lasers Med. Sci. 34 (2019) 1193–1200
[43] S. Young, P. Bolton, M. Dyson, W. Harvey, C. Diamantopoulos, “Macrophage responsiveness to light therapy Lasers Surg”, Med. 9 (1989) 497–505.
[44] S.K. Kim, H.R. You, S.H. Kim, S.J. Yun, S.C. Lee, J.B. Lee, “Skin photorejuvenation effects of light-emitting diodes (LEDs): a comparative study of yellow and red LEDs in vitro and in vivo, Clin. Exp”. Dermatol. 41 (2016) 798–805.
[45] B.A. Russell, N. Kellett, L.R. Reilly, “A study to determine the efficacy of
combination LED light therapy (633 nm and 830 nm) in facial skin rejuvenation” J. Cosmet. Laser. Ther. 7 (2005) 196–200.
[46] H.E. Lee, S.H. Lee, M. Jeong, J.H. Shin, Y. Ahn, D. Kim, S.H. Oh, S.H. Yun, K.J. Lee, “Trichogenic photostimulation using monolithic flexible vertical Algainp light emitting diodes”, ACS Nano 12 (2018) 9587–9595.[47] Z. Santos, P. Avci, M.R. Hamblin, “Drug discovery for alopecia: gone today, hair tomorrow, Expert Opin”. Drug Discov. 10 (2015) 269–292.
[48] P. Avci, T.T. Nyame, G.K. Gupta, M. Sadasivam, M.R. Hamblin, “Low-level laser therapy for fat layer reduction: a comprehensive review”, Laser Surg. Med. 45 (2013) 349–357
[49] G. Silva, C. Ferraresi, R.T.D. Almeida, M.L. Motta, T. Paixao, ˜ V.O. Ottone, I.A. Fonseca, M.X. Oliveira, E. Rocha-Vieira, M.F. Dias-Peixoto, E.A. Esteves, C.
C. Coimbra, F.T. Amorim, F.D.C. Magalhaes, “ Infrared photobiomodulation (PBM) therapy improves glucose metabolism and intracellular insulin pathway in adipose tissue of high-fat fed mice”, Lasers Med. Sci. 33 (2018) 559–571.
[50] F.R. Paolillo, A. Borghi-Silva, N.A. Parizotto, C. Kurachi, V.S. Bagnato, “New
treatment of cellulite with infrared-LED illumination applied during highintensity treadmill training”, J. Cosmet. Laser Ther. 13 (2011) 166–171.
[51] A.A. Vanin, E. Verhagen, S.D. Barboza, L.O.P. Costa, E.C.P. Leal-Junior,
“Photobiomodulation therapy for the improvement of muscular performance and reduction of muscular fatigue associated with exercise in healthy people: a
systematic review and meta-analysis”, Lasers Med. Sci. 33 (2018) 181–214
[52] Andrea L. Looney, Janice L. Huntingford, Lauren L. Blaeser, Sabine Mann “A randomized blind placebo-controlled trial investigating the effects of
photobiomodulation therapy (PBMT) on canine elbow osteoarthritis”, Can Vet J 2018;59:959–966
[53] Solange Almeida dos Santos, Andrey Jorge Serra, Tatiane Garcia Stancker,
Maíra Cecília Brandão Simões, Marcia Ataíze dos Santos Vieira,
Ernesto Cesar Leal-Junior, Marko Prokic, Andrea Vasconsuelo, Simone Silva Santos, and Paulo de Tarso Camillo de Carvalho, “Effects of Photobiomodulation Therapy on Oxidative Stress in Muscle Injury Animal Models: A Systematic Review” Hindawi. Oxidative Medicine and Cellular Longevity Volume 2017, Article ID 5273403
[54] Mira M. Mandelbaum-Livnat, PhD, Mara Almog, PhD, Moshe Nissan, PhD,
Emmanuel Loeb, DVM, Yuval Shapira, MD, and Shimon Rochkind, MD, PhD “Photobiomodulation Triple Treatment in Peripheral Nerve Injury: Nerve and Muscle Response” Photomedicine and Laser Surgery Volume 34, Number 12, 2016
[55] Marcelie Priscila de Oliveira Rosso, Daniela Vieira Buchaim, Natália Kawano, Gabriela Furlanette, Karina Torres Pomini and Rogério Leone Buchaim “Photobiomodulation Therapy (PBMT) in Peripheral Nerve Regeneration: A Systematic Review” Bioengineering 2018, 5, 44; doi:10.3390/bioengineering5020044
[56] Giovanna Moura Lamas Della Santa, Marcılio Coelho Ferreira,Thaıs Peixoto Gaiad Machado, Murilo Xavier Oliveira, and Ana Paula Santos “Effects of Photobiomodulation Therapy (LED 630 nm) on Muscle and Nerve Histomorphometry after Axonotmesis”. Photochemistry and Photobiology, 2021, DOI: 10.1111/php.13415
[57] Hamblin MR “Mechanisms and mitochondrial redox signaling in photobiomodulation”. Photochem Photobiol 2018 94(2):199–212
[58] Luana Gabriel de Souza1 , Alexandre Márcio Marcolino1 , Heloyse Uliam Kuriki1 , Elaine Cristina Dalazen Gonçalves2 , Marisa de Cássia Registro Fonseca3 , Rafael Inácio Barbosa, “Comparative effect of photobiomodulation associated with dexamethasone after sciatic nerve injury model”. Springer-Verlag London Ltd., part of Springer Nature 2018
[59] Vahid Mansouri, Babak Arjmand, Mostafa Rezaei Tavirani, Mohammadreza Razzaghi, Mohammad Rostami-Nejad, Mostafa Hamdieh “Evaluation of Efficacy of Low-Level Laser Therapy”.J Lasers Med Sci 2020 Autumn;11(4):369-380
[60] Tezcan S, Ulu Ozturk F, Uslu N, Nalbant M, Umit Yemisci O. “Carpal tunnel syndrome: evaluation of the effects of low‐level laser therapy with ultrasound strain imaging”. J Ultrasound Med. 2019;38(1):113-22. doi: 10.1002/ jum.14669
[61] Jannani T.P Prathap Suganthirababu Kumaresan A Vignesh Srinivasan Divyalaxmi P Aravind Ganesh Swetha Hari “Effect of Low- Level Laser Therapy in the management of Carpal Tunnel Syndrome – A Systematic Review” European Journal of Molecular and Clinical Medicine (EJMCM) ISSN: 2515-8260 Volume 09, Issue 08, 2022
[62] Barbosa et al., “Effectiveness of low-level laser therapy for patients with carpal tunnel syndrome: Design of a randomized single-blinded controlled trial”. BMC musculoskeletal disorders. (2012). 13. 248. 10.1186/1471-2474-13-248.
[63] Lazovic, Milica,IlicStojanovic et al. “Placebo-Controlled Investigation of LowLevel Laser Therapy to Treat Carpal Tunnel Syndrome”. Photomedicine and laser surgery(2014). 32.336-344.
[64] Tikiz, Canan&Duruoz et al., (2013). “Comparison of the Efficacy of Low-Level Laser Therapy and Pulsed Ultrasound Treatment in Carpal Tunnel Syndrome: A Placebo-Controlled Study”. TurkiyeFiziksel Tip veRehabilitasyonDergisi. 59. 201-208. 10.4274/tftr.04764.
[65] Buchaim, R.L.; Andreo, J.C.; Barraviera, B.; Ferreira Junior, R.S.; Buchaim, D.V.; Rosa Junior, G.M.; de Oliveira, A.L.; de Castro Rodrigues, A. “Effect of low-level laser therapy (LLLT) on peripheral nerve regeneration using fibrin glue derived from snake venom”. Injury 2015, 46, 655–660.
[66] Takhtfooladi, M.A.; Jahanbakhsh, F.; Takhtfooladi, H.A.; Yousefi, K.; Allahverdi, A. “Effect of low-level laser therapy (685 nm, 3 J/cm2) on functional recovery of the sciatic nerve in rats following crushing lesion”.Lasers Med. Sci. 2015, 30, 1047–1052.
[67] Wang, C.Z.; Chen, Y.J.; Wang, Y.H.; Yeh, M.L.; Huang, M.H.; Ho, M.L.; Liang, J.I.; Chen, C.H. “Low-level laser irradiation improves functional recovery and nerve regeneration in sciatic nerve crush rat injury model”.PLoS ONE 2014, 13, e103348.
[68] Shen, C.C.; Yang, Y.C.; Huang, T.B.; Chan, S.C.; Liu, B.S. “Low-Level Laser-Accelerated Peripheral Nerve Regeneration within a Reinforced Nerve Conduit across a Large Gap of the Transected Sciatic Nerve in Rats”. Evid. Based Complement. Altern. Med. 2013, 2013, 175629.
[69] Marcolino, A.M.; Barbosa, R.I.; das Neves, L.M.; Mazzer, N.; de Jesus Guirro, R.R.; de Cássia Registro Fonseca, M. “Assessment of functional recovery of sciatic nerve in rats submitted to low-level laser therapy with different fluences”. An experimental study: Laser in functional recovery in rats. J. Hand Microsurg. 2013,
5, 49–53.
[70] Sene, G.A.; Sousa, F.F.; Fazan, V.S.; Barbieri, C.H. “Effects of laser therapy in peripheral nerve regeneration”. Acta Ortop. Bras. 2013, 21, 266–270.
[71] Dias, F.J.; Issa, J.P.; Coutinho-Netto, J.; Fazan, V.P.; Sousa, L.G.; Iyomasa, M.M.; Papa, P.C.; Watanabe, I.S. “Morphometric and high-resolution scanning electron microscopy analysis of low-level laser therapy and latex protein (Hevea brasiliensis) administration following a crush injury of the sciatic nerve in rats”. J. Neurol. Sci. 2015, 349, 129–137.
[72] Ziago, E.K.; Fazan, V.P.; Iyomasa, M.M.; Sousa, L.G.; Yamauchi, P.Y.; da Silva, E.A.; Borie, E.; Fuentes, R.; Dias, F.J. “Analysis of the variation in low-level laser energy density on the crushed sciatic nerves of rats: A morphological, quantitative, and morphometric study”. Lasers Med. Sci. 2017, 32, 369–378.
[73] Yongmin Jeon, Hye-Ryung Choi, Myungsub Lim, Seungyeop Choi, Hyuncheol Kim, Jeong Hyun Kwon, Kyoung-Chan Park, and Kyung Cheol Choi. “A Wearable Photobiomodulation Patch Using a Flexible Red-Wavelength OLED and Its In Vitro Differential Cell Proliferation Effects” Adv. Mater. Technol. 2018, 1700391
[74] P. Suchonwanit, N. Chalermroj, S. Khunkhet, “Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: a 24-week,
randomized, double-blind, sham device-controlled trial”, Lasers Med. Sci. 1–8
(2018)
[75] H. Kim, J.W. Choi, J.Y. Kim, J.W. Shin, S.-J. Lee, C.-H. Huh, “Low-Level Light
Therapy for Androgenetic Alopecia: A 24-Week, Randomized, Double-Blind,
Sham Device-Controlled Multicenter Trial, Dermatol”. Surg. 39 (2013)
1177–1183.
[76] T.S. Hamid, Portable light hair restoration helmet, United States (2012)