Introduction
Interoception is the body’s ability to detect and interpret interior bodily sensations, which provides details on the overall physiological state of the body. Originally defined as sensations originating from within the body, such as cardiac, respiratory, and digestive functions, Interoception has been redefined and extended to include signals from the body surface, like temperature, itch, pain, and pleasure from sensual touch. These signals are conveyed through specialized afferent pathways. Interoception plays a role in generating bodily feelings and informing the organism about its bodily needs [1].
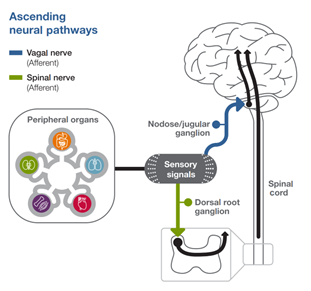
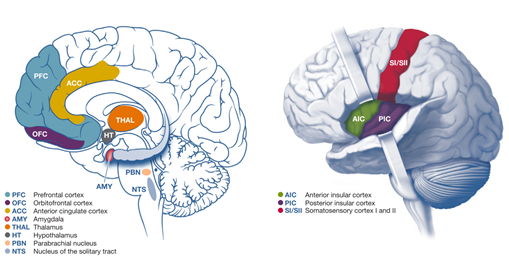
Interoceptive information is initially processed in subcortical structures of the brain, such as the medial nucleus tractus solitarii, the parabrachial nucleus, and the ventromedial nucleus of the thalamus. For more integration and interpretation, it is afterward projected to higher brain areas such as the hypothalamus, insula, anterior cingulate cortex, and somatosensory cortex Figure 2 [3].
Interoception plays a vital role in monitoring and regulating the body’s internal state to achieve homeostasis or Allostasis in response to the complex environment [4]. Homeostasis, which involves the regulation of internal conditions for stability, relies heavily on Interoception. Interoceptive signals act as the means to detect and evaluate deviations from the body’s optimal internal states. When there is a discrepancy between the actual internal state and the desired setpoint, interoceptive feedback triggers corrective actions to restore equilibrium [5].
On the other hand, Allostasis is the process by which the brain anticipates and responds to the changing needs of the body, aiming to maintain internal stability and adapt to environmental demands. It involves the activation of various physiological systems, such as the hormonal, autonomic, and immune systems, to restore the body to a state of balance or homeostasis. So, it means keeping the body’s parameters in a relatively stable range. This adaptive response to physiological and environmental challenges is supported by Interoception, which provides real-time information about the body’s internal states [6] [7] [8].
Breathing and interoception
Respiratory interoception, also known as respiroception, encompasses a rich blend of sensory cues originating from various sources within and outside the body including the contraction of the diaphragm’s smooth muscle, monitoring of blood oxygen and carbon dioxide levels, respiratory frequency, activation of stretch and baroreceptors distributed across the neck and chest [9].
The respiratory domain is a complex mix of interoceptive and exteroceptive sensory-motor and chemo-sensory channels. During normal ventilation, the diaphragm’s distension and contraction mechanically pump air into the lungs, creating a rhythmic pattern. As air flows through the mouth, nose, and upper airway, thermosensory and tactile receptors play a role in conveying this external information. Simultaneously, stretch receptors located in the diaphragm, chest wall, and surrounding organs activate in response to the rhythmic expansion and contraction of the lungs [9].
Sensory information from the respiratory domain is transmitted to multiple regions within the brain. Medullary and pontine brainstem nuclei and the somatosensory cortex receive these signals and play important roles in processing respiratory sensations. Additionally, higher-order structures like the insula are involved in integrating respiratory perception with cognitive and affective processes. This intricate interplay of neural pathways and higher-order processing allows us to comprehend the sensations associated with our breath and connect them to our overall sensory experiences [10, 11].
Predictive Coding Model of Respiratory Interoception
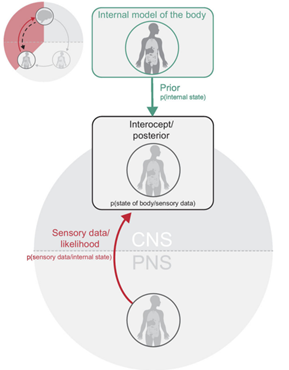
The brain utilizes a paradigm known as predictive processing to understand its interactions with the body. According to this framework, the brain constructs generative models that anticipate the effects of changes in the environment or the body on the organism. These models are learned based on past experiences. The brain then compares the sensory information it receives with its own predictions. When there is a mismatch between the predictions and the actual observations, a prediction error occurs. For reducing prediction errors, the organism has two different option. By altering its sensory states through activities to update its predictions, it can engage in active inference. As an alternative, it can use a process known as perceptual inference to modify its internal dynamics to more closely fit the surrounding circumstances [6].
Predictive processing in the nervous system is achieved through hierarchical predictive coding, which computes prediction errors between prior beliefs and sensory inputs [12]. It operates by generating top-down predictions based on prior knowledge and comparing them with bottom-up sensory signals to generate prediction errors. These prediction errors are then used to update and refine the predictions, reducing uncertainty [13] [14] [15]. This hierarchical organization within the brain enables efficient information processing, as prediction errors propagate up the hierarchy to update higher-level predictions. Within this framework, Interoception is treated as prediction errors, allowing the brain to maintain a coherent model of the body and its environment. [16] [17] [18]
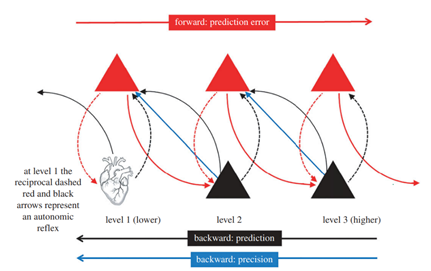
Figure 3 provides a schematic overview of the hierarchical message passing in the brain. The figure illustrates the flow of predictions and prediction errors (PEs) through the hierarchy of the brain. Predictions are represented by black lines and are projected downward from prediction units (deep pyramidal cells) depicted as black triangles. On the other hand, prediction errors are represented by red lines and pass upward from prediction error units (superficial pyramidal cells) shown as red triangles. Importantly, the Figure demonstrates that predictions and prediction errors occur at every level of the hierarchy. The dashed red and black arrows indicate local processing within a level. At the lowest level (level 1), they represent an autonomic reflex. One important aspect depicted in the figure is the role of precision, indicated by the blue arrows. The precision determines the relative weight of prediction errors compared to priors at each level of the hierarchy. Precision is believed to play a critical role in updating and refining predictions based on incoming sensory information.
As previously mentioned, the overall goal of this hierarchical message passing is to minimize prediction errors at all levels within the hierarchy, resulting in the formation of a percept. The iterative process of updating predictions based on prediction errors contributes to the construction of an accurate representation of the external world and the internal states of the organism [19].
Allostasis, the process of adaptively altering physiological parameters of the body with respect to environmental changes, is the main objective of predictive processing within the nervous system. Allostasis achieves homeostasis, which maintains physiological parameters within a relatively constant range, by ensuring that the organism adapts to a precarious environment. The management of these parameters requires constant handling of immense amounts of information, which the nervous system achieves by employing a clever strategy: instead of processing all of the information, it only registers the discrepancies from what was expected. Thus, the brain-centered predictive regulation of allostasis coordinates changes in bodily systems to assure that the organism is prepared to meet the body’s basic needs, such as food, warmth, and cooling, before they are at stake, to keep the system in equilibrium.
Changes in rate, depth, and duration of breathing serve as dynamic regulatory mechanisms, driven by the coordinated activity of neuronal oscillations, allowing the organism to downregulate or upregulate its system, resulting in distinct cognitive, affective, and physiological changes. These respiratory-driven neuronal oscillations synchronize neural activity across different brain regions, facilitating the integration of sensory information and the generation of predictions. By actively updating top-down interoceptive predictions based on bottom-up sensory evidence, controlled breathing enables the organism to adapt to environmental changes and achieve allostasis [20]. This process involves the interplay between neuronal oscillations and the generation of prediction errors (PEs). Whenever there is a mismatch between predicted and observed sensory information, PEs propagate back to cortical regions, triggering adjustments in neuronal oscillatory patterns. The organism then employs active inference, modifying its sensory states through actions or adjusting internal dynamics to minimize PEs and optimize predictions, a process known as perceptual inference. The intricate interplay between respiratory-driven neuronal oscillations, predictive processing, and perceptual inference provides a foundation for understanding how controlled breathing shapes neural dynamics and facilitates adaptive responses to a dynamic environment [12, 21].
Respiration stands apart from other interoceptive modalities due to its distinct characteristic: our conscious control over this domain and its potential to influence other interoceptive variables, such as cardiac arousal. This ability opens the door to respiratory-brain coupling, which emerges as a unique domain for respiratory active inference. In this process, individuals actively monitor and regulate their breathing patterns, to optimize prediction error in response to the variable environment. This optimized regulation of respiratory dynamics allows for the synchronization of the breath with specific cognitive and affective states, thereby optimizing performance and well-being. Various traditional mindfulness and meditation practices, which emphasize working with the breath, provide invaluable insights into this process of respiratory allostasis. Mindfulness and meditation techniques have long recognized the interplay between respiration and optimal functioning. Intentionally focusing on and manipulating the breath help individuals to gain the ability to regulate their internal states and bring about a harmonious balance. Through precise modulation of breathing patterns, practitioners can induce a state of relaxation, heightened attention, or increased energy and alertness [22, 23].
To provide an example of how controlled breathing and predictive processing interact, let’s consider a stressful public speaking scenario. When faced with the task of delivering a speech in front of a large audience, individuals often experience heightened anxiety and a surge of physiological responses such as increased heart rate, rapid breathing, and tense muscles. However, by consciously attending to the breath and engaging in slow, deep breathing techniques, it is possible to modulate the internal state and shift from a state of anxiety to a more composed and focused state, all within the same external circumstances.
As individuals focus their attention on the breath, they initiate a cascade of physiological responses that influence the autonomic nervous system. Slow and deliberate inhalation activates the parasympathetic branch of the autonomic nervous system, which is responsible for promoting relaxation and restoring the body to a state of balance. This activation helps to counteract the effects of the sympathetic branch, which is associated with the fight-or-flight response. controlled breathing introduces new sensory inputs that deviate from the brain’s predictions. These bottom-up respiratory signals, signaling a calm and relaxed state, feed into the predictive processing loop. This discrepancy between the predicted and actual sensory inputs generates prediction errors, which prompt the brain to update its internal models and adjust the expectations.
Through this process of active inference, individuals set new homeostatic setpoints and optimize prediction error minimization in the new context. By modulating their breathing and providing sensory evidence of relaxation, they effectively reshape the interoceptive predictions, leading to a shift in the cognitive and emotional state. The heightened anxiety dissipates, and individuals enter a more composed and focused state, better equipped to deliver their speech with confidence and clarity.
References
- Crucianelli, L., A. Enmalm, and H.H. Ehrsson, Probing interoception via thermosensation: No specific relationships across multiple interoceptive sub-modalities. bioRxiv, 2021: p. 2021.03. 04.433866.
- Chen, W.G., et al., The emerging science of interoception: sensing, integrating, interpreting, and regulating signals within the self. Trends in neurosciences, 2021. 44(1): p. 3-16.
- Salomon, R., et al., The insula mediates access to awareness of visual stimuli presented synchronously to the heartbeat. Journal of Neuroscience, 2016. 36(18): p. 5115-5127.
- Ueno, D., H. Ohira, and J. Narumoto, Interoception and the autonomic nervous system: Investigating affect, decision-making, and mental health. 2023, Frontiers Media SA. p. 1130324.
- Sel, A., Predictive codes of interoception, emotion, and the self. Frontiers in psychology, 2014. 5: p. 189.
- Boyadzhieva, A. and E. Kayhan, Keeping the breath in mind: Respiration, neural oscillations, and the free energy principle. Frontiers in Neuroscience, 2021: p. 742.
- Katsumi, Y., et al., Allostasis as a core feature of hierarchical gradients in the human brain. Network Neuroscience, 2022. 6(4): p. 1010-1031.
- Sterling, P., Allostasis: a model of predictive regulation. Physiology & behavior, 2012. 106(1): p. 5-15.
- Schoeller, F., et al., Interoceptive technologies for clinical neuroscience. 2022, PsyArXiv.
- Allen, M., et al., In the Body’s Eye: The computational anatomy of interoceptive inference. PLoS Comput Biol, 2022. 18(9): p. e1010490.
- Kluger, D.S., et al., Respiration aligns perception with neural excitability. eLife, 2021. 10: p. e70907.
- Allen, M., S. Varga, and D.H. Heck, Respiratory rhythms of the predictive mind. Psychol Rev, 2023. 130(4): p. 1066-1080.
- Nikolova, N., et al., What might interoceptive inference reveal about consciousness? Review of Philosophy and Psychology, 2022. 13(4): p. 879-906.
- Millidge, B., A. Seth, and C.L. Buckley, Predictive coding: a theoretical and experimental review. arXiv preprint arXiv:2107.12979, 2021.
- Williams, D., Predictive coding and thought. Synthese, 2020. 197(4): p. 1749-1775.
- Barrett, L.F. and W.K. Simmons, Interoceptive predictions in the brain. Nature reviews neuroscience, 2015. 16(7): p. 419-429.
- Seth, A.K. and K.J. Friston, Active interoceptive inference and the emotional brain. Philosophical Transactions of the Royal Society B: Biological Sciences, 2016. 371(1708): p. 20160007.
- Seth, A.K. and M. Tsakiris, Being a beast machine: The somatic basis of selfhood. Trends in cognitive sciences, 2018. 22(11): p. 969-981.
- Ainley, V., et al., ‘Bodily precision’: a predictive coding account of individual differences in interoceptive accuracy. Philosophical Transactions of the Royal Society B: Biological Sciences, 2016. 371(1708): p. 20160003.
- Boyadzhieva, A. and E. Kayhan, Keeping the Breath in Mind: Respiration, Neural Oscillations, and the Free Energy Principle. Front Neurosci, 2021. 15: p. 647579.
- Brændholt, M., et al., Breathing in waves: Understanding respiratory-brain coupling as a gradient of predictive oscillations. Neuroscience & Biobehavioral Reviews, 2023. 152: p. 105262.
- Nikolova, N., et al., The respiratory resistance sensitivity task: An automated method for quantifying respiratory interoception and metacognition. Biol Psychol, 2022. 170: p. 108325.
- Zelano, C., et al., Nasal Respiration Entrains Human Limbic Oscillations and Modulates Cognitive Function. J Neurosci, 2016. 36(49): p. 12448-12467.
- Petzschner, F.H., et al., Computational models of interoception and body regulation. Trends in neurosciences, 2021. 44(1): p. 63-76.